βA3/A1-crystallin and persistent fetal vasculature (PFV) disease of the eye
- PMID: 26022148
- PMCID: PMC4666823
- DOI: 10.1016/j.bbagen.2015.05.017
βA3/A1-crystallin and persistent fetal vasculature (PFV) disease of the eye
Abstract
Background: Persistent fetal vasculature (PFV) is a human disease in which the fetal vasculature of the eye fails to regress normally. The fetal, or hyaloid, vasculature nourishes the lens and retina during ocular development, subsequently regressing after formation of the retinal vessels. PFV causes serious congenital pathologies and is responsible for as much as 5% of blindness in the United States.
Scope of review: The causes of PFV are poorly understood, however there are a number of animal models in which aspects of the disease are present. One such model results from mutation or elimination of the gene (Cryba1) encoding βA3/A1-crystallin. In this review we focus on the possible mechanisms whereby loss of functional βA3/A1-crystallin might lead to PFV.
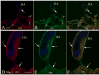
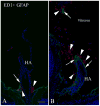
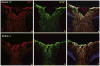
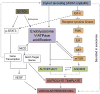
Major conclusions: Cryba1 is abundantly expressed in the lens, but is also expressed in certain other ocular cells, including astrocytes. In animal models lacking βA3/A1-crystallin, astrocyte numbers are increased and they migrate abnormally from the retina to ensheath the persistent hyaloid artery. Evidence is presented that the absence of functional βA3/A1-crystallin causes failure of the normal acidification of endolysosomal compartments in the astrocytes, leading to impairment of certain critical signaling pathways, including mTOR and Notch/STAT3.
General significance: The findings suggest that impaired endolysosomal signaling in ocular astrocytes can cause PFV disease, by adversely affecting the vascular remodeling processes essential to ocular development, including regression of the fetal vasculature. This article is part of a Special Issue entitled Crystallin Biochemistry in Health and Disease.
Keywords: Astrocytes; Fetal/hyaloid vasculature; Notch/STAT signaling; PI3K/Akt/mTOR signaling; Vascular remodeling; βA3/A1-crystallin.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures



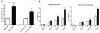


Similar articles
-
Modulating EGFR-MTORC1-autophagy as a potential therapy for persistent fetal vasculature (PFV) disease.Autophagy. 2020 Jun;16(6):1130-1142. doi: 10.1080/15548627.2019.1660545. Epub 2019 Sep 1. Autophagy. 2020. PMID: 31462148 Free PMC article.
-
βA3/A1-crystallin: more than a lens protein.Prog Retin Eye Res. 2015 Jan;44:62-85. doi: 10.1016/j.preteyeres.2014.11.002. Epub 2014 Nov 13. Prog Retin Eye Res. 2015. PMID: 25461968 Free PMC article. Review.
-
βA3/A1-crystallin is required for proper astrocyte template formation and vascular remodeling in the retina.Transgenic Res. 2012 Oct;21(5):1033-42. doi: 10.1007/s11248-012-9608-0. Epub 2012 Mar 17. Transgenic Res. 2012. PMID: 22427112 Free PMC article.
-
βA3/A1-Crystallin controls anoikis-mediated cell death in astrocytes by modulating PI3K/AKT/mTOR and ERK survival pathways through the PKD/Bit1-signaling axis.Cell Death Dis. 2011 Oct 13;2(10):e217. doi: 10.1038/cddis.2011.100. Cell Death Dis. 2011. PMID: 21993393 Free PMC article.
-
Persistent Fetal Vasculature.Asia Pac J Ophthalmol (Phila). 2019 Jan-Feb;8(1):86-95. doi: 10.22608/APO.201854. Epub 2018 Oct 30. Asia Pac J Ophthalmol (Phila). 2019. PMID: 30375202 Review.
Cited by
-
Bidirectional Analysis of Cryba4-Crybb1 Nascent Transcription and Nuclear Accumulation of Crybb3 mRNAs in Lens Fibers.Invest Ophthalmol Vis Sci. 2019 Jan 2;60(1):234-244. doi: 10.1167/iovs.18-25921. Invest Ophthalmol Vis Sci. 2019. PMID: 30646012 Free PMC article.
-
Regulation of the rhythmic diversity of daily photoreceptor outer segment phagocytosis in vivo.FASEB J. 2022 Oct;36(10):e22556. doi: 10.1096/fj.202200990RR. FASEB J. 2022. PMID: 36165194 Free PMC article. Review.
-
Generation of Lens Progenitor Cells and Lentoid Bodies from Pluripotent Stem Cells: Novel Tools for Human Lens Development and Ocular Disease Etiology.Cells. 2022 Nov 6;11(21):3516. doi: 10.3390/cells11213516. Cells. 2022. PMID: 36359912 Free PMC article. Review.
-
Immunohistochemical Detection of Encephalitozoon cuniculi in Ocular Structures of Immunocompetent Rabbits.Animals (Basel). 2019 Nov 18;9(11):988. doi: 10.3390/ani9110988. Animals (Basel). 2019. PMID: 31752146 Free PMC article.
-
Heat Shock Proteins Regulatory Role in Neurodevelopment.Front Neurosci. 2018 Nov 12;12:821. doi: 10.3389/fnins.2018.00821. eCollection 2018. Front Neurosci. 2018. PMID: 30483047 Free PMC article. Review.
References
-
- Goldberg MF. Persistent fetal vasculature (PFV): an integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (PHPV). LIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 1997;124:587–626. - PubMed
-
- Zhang C, Ashaghi L, Gongora C, Patek B, Hose S, Ma B, Aghsaei-Fard M, Brako L, Singh K, Goldberg MF, Handa JT, Lo WK, Eberhart CG, Zigler JS, Jr, Sinha D. A developmental defect in astrocytes inhibits programmed regression of the hyaloid vasculature in the mammalian eye. Eur J Cell Biol. 2011;90(5):440–448. - PMC - PubMed
-
- Zhang C, Gehlbach P, Gongora C, Cano M, Fariss R, Hose S, Nath A, Green WR, Goldberg MF, Zigler JS, Sinha D. A potential role for β-and γ-crystallins in the vascular remodeling of the eye. Dev Dyn. 2005;234:36–47. - PubMed
-
- Ito M, Yoshioka M. Regression of the hyaloid vessels and pupillary membrane of the mouse. Anat Embryol. 1999;200:403–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

