Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in Marchantia polymorpha
- PMID: 26020919
- PMCID: PMC4447296
- DOI: 10.1371/journal.pgen.1005084
Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in Marchantia polymorpha
Erratum in
-
Correction: Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in Marchantia polymorpha.PLoS Genet. 2015 Jun 26;11(6):e1005365. doi: 10.1371/journal.pgen.1005365. eCollection 2015 Jun. PLoS Genet. 2015. PMID: 26115224 Free PMC article. No abstract available.
Abstract
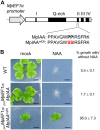
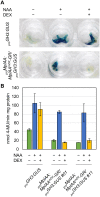
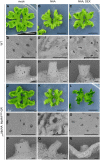
The plant hormone auxin regulates many aspects of plant growth and development. Recent progress in Arabidopsis provided a scheme that auxin receptors, TIR1/AFBs, target transcriptional co-repressors, AUX/IAAs, for degradation, allowing ARFs to regulate transcription of auxin responsive genes. The mechanism of auxin-mediated transcriptional regulation is considered to have evolved around the time plants adapted to land. However, little is known about the role of auxin-mediated transcription in basal land plant lineages. We focused on the liverwort Marchantia polymorpha, which belongs to the earliest diverging lineage of land plants. M. polymorpha has only a single TIR1/AFB (MpTIR1), a single AUX/IAA (MpIAA), and three ARFs (MpARF1, MpARF2, and MpARF3) in the genome. Expression of a dominant allele of MpIAA with mutations in its putative degron sequence conferred an auxin resistant phenotype and repressed auxin-dependent expression of the auxin response reporter proGH3:GUS. We next established a system for DEX-inducible auxin-response repression by expressing the putatively stabilized MpIAA protein fused with the glucocorticoid receptor domain (MpIAA(mDII)-GR). Repression of auxin responses in (pro)MpIAA:MpIAA(mDII)-GR plants caused severe defects in various developmental processes, including gemmaling development, dorsiventrality, organogenesis, and tropic responses. Transient transactivation assays showed that the three MpARFs had different transcriptional activities, each corresponding to their phylogenetic classifications. Moreover, MpIAA and MpARF proteins interacted with each other with different affinities. This study provides evidence that pleiotropic auxin responses can be achieved by a minimal set of auxin signaling factors and suggests that the transcriptional regulation mediated by TIR1/AFB, AUX/IAA, and three types of ARFs might have been a key invention to establish body plans of land plants. We propose that M. polymorpha is a good model to investigate the principles and the evolution of auxin-mediated transcriptional regulation and its roles in land plant morphogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort Marchantia polymorpha.PLoS Genet. 2015 May 28;11(5):e1005207. doi: 10.1371/journal.pgen.1005207. eCollection 2015 May. PLoS Genet. 2015. PMID: 26020649 Free PMC article.
-
Class C ARFs evolved before the origin of land plants and antagonize differentiation and developmental transitions in Marchantia polymorpha.New Phytol. 2018 Jun;218(4):1612-1630. doi: 10.1111/nph.15090. Epub 2018 Mar 25. New Phytol. 2018. PMID: 29574879
-
Auxin sensitivities of all Arabidopsis Aux/IAAs for degradation in the presence of every TIR1/AFB.Plant Cell Physiol. 2014 Aug;55(8):1450-9. doi: 10.1093/pcp/pcu077. Epub 2014 May 31. Plant Cell Physiol. 2014. PMID: 24880779
-
SCFTIR1/AFB-based auxin perception: mechanism and role in plant growth and development.Plant Cell. 2015 Jan;27(1):9-19. doi: 10.1105/tpc.114.133744. Epub 2015 Jan 20. Plant Cell. 2015. PMID: 25604443 Free PMC article. Review.
-
Evolution of nuclear auxin signaling: lessons from genetic studies with basal land plants.J Exp Bot. 2018 Jan 4;69(2):291-301. doi: 10.1093/jxb/erx267. J Exp Bot. 2018. PMID: 28992186 Review.
Cited by
-
Auxin perception and downstream events.Curr Opin Plant Biol. 2016 Oct;33:8-14. doi: 10.1016/j.pbi.2016.04.004. Epub 2016 Apr 27. Curr Opin Plant Biol. 2016. PMID: 27131035 Free PMC article. Review.
-
R2R3-MYB transcription factor GEMMA CUP-ASSOCIATED MYB1 mediates the cytokinin signal to achieve proper organ development in Marchantia polymorpha.Sci Rep. 2022 Dec 7;12(1):21123. doi: 10.1038/s41598-022-25684-3. Sci Rep. 2022. PMID: 36477255 Free PMC article.
-
Co-expression and Transcriptome Analysis of Marchantia polymorpha Transcription Factors Supports Class C ARFs as Independent Actors of an Ancient Auxin Regulatory Module.Front Plant Sci. 2018 Oct 1;9:1345. doi: 10.3389/fpls.2018.01345. eCollection 2018. Front Plant Sci. 2018. PMID: 30327658 Free PMC article.
-
Auxin Response Factors promote organogenesis by chromatin-mediated repression of the pluripotency gene SHOOTMERISTEMLESS.Nat Commun. 2019 Feb 21;10(1):886. doi: 10.1038/s41467-019-08861-3. Nat Commun. 2019. PMID: 30792395 Free PMC article.
-
Plant science's next top models.Ann Bot. 2020 Jun 19;126(1):1-23. doi: 10.1093/aob/mcaa063. Ann Bot. 2020. PMID: 32271862 Free PMC article. Review.
References
-
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, et al. (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153. - PubMed
-
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, et al. (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602. - PubMed
-
- Tatematsu K, Kumagai S, Muto H, Sato A, Watahiki MK, et al. (2004) MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell 16: 379–393. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

