Modulation of voltage-gated Ca2+ channels by G protein-coupled receptors in celiac-mesenteric ganglion neurons of septic rats
- PMID: 26017846
- PMCID: PMC4446366
- DOI: 10.1371/journal.pone.0125566
Modulation of voltage-gated Ca2+ channels by G protein-coupled receptors in celiac-mesenteric ganglion neurons of septic rats
Abstract
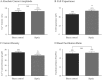
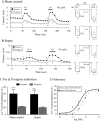
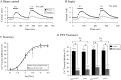
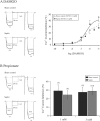
Septic shock, the most severe complication associated with sepsis, is manifested by tissue hypoperfusion due, in part, to cardiovascular and autonomic dysfunction. In many cases, the splanchnic circulation becomes vasoplegic. The celiac-superior mesenteric ganglion (CSMG) sympathetic neurons provide the main autonomic input to these vessels. We used the cecal ligation puncture (CLP) model, which closely mimics the hemodynamic and metabolic disturbances observed in septic patients, to examine the properties and modulation of Ca2+ channels by G protein-coupled receptors in acutely dissociated rat CSMG neurons. Voltage-clamp studies 48 hr post-sepsis revealed that the Ca2+ current density in CMSG neurons from septic rats was significantly lower than those isolated from sham control rats. This reduction coincided with a significant increase in membrane surface area and a negligible increase in Ca2+ current amplitude. Possible explanations for these findings include either cell swelling or neurite outgrowth enhancement of CSMG neurons from septic rats. Additionally, a significant rightward shift of the concentration-response relationship for the norepinephrine (NE)-mediated Ca2+ current inhibition was observed in CSMG neurons from septic rats. Testing for the presence of opioid receptor subtypes in CSMG neurons, showed that mu opioid receptors were present in ~70% of CSMG, while NOP opioid receptors were found in all CSMG neurons tested. The pharmacological profile for both opioid receptor subtypes was not significantly affected by sepsis. Further, the Ca2+ current modulation by propionate, an agonist for the free fatty acid receptors GPR41 and GPR43, was not altered by sepsis. Overall, our findings suggest that CSMG function is affected by sepsis via changes in cell size and α2-adrenergic receptor-mediated Ca2+ channel modulation.
Conflict of interest statement
Figures

Similar articles
-
Gα14 subunit-mediated inhibition of voltage-gated Ca2+ and K+ channels via neurokinin-1 receptors in rat celiac-superior mesenteric ganglion neurons.J Neurophysiol. 2016 Mar;115(3):1577-86. doi: 10.1152/jn.00980.2015. Epub 2016 Feb 3. J Neurophysiol. 2016. PMID: 26843606 Free PMC article.
-
Nociceptin receptor signaling in sympathetic neurons from septic rats.J Surg Res. 2013 Oct;184(2):973-80. doi: 10.1016/j.jss.2013.03.076. Epub 2013 Apr 11. J Surg Res. 2013. PMID: 23608620 Free PMC article.
-
TTX-sensitive Na+ channels and Ca2+ channels of the L- and N-type underlie the inward current in acutely dispersed coeliac-mesenteric ganglia neurons of adult rats.Pflugers Arch. 1992 May;421(1):7-16. doi: 10.1007/BF00374726. Pflugers Arch. 1992. PMID: 1321408
-
Modulation of Ca2+ currents by various G protein-coupled receptors in sympathetic neurons of male rat pelvic ganglia.J Neurophysiol. 1997 Aug;78(2):780-9. doi: 10.1152/jn.1997.78.2.780. J Neurophysiol. 1997. PMID: 9307112
-
alpha 2-Adrenoreceptor-mediated inhibition of acetylcholine-induced noradrenaline release from rat sympathetic neurons: an action at voltage-gated Ca2+ channels.Neuroscience. 1995 Nov;69(1):221-31. doi: 10.1016/0306-4522(95)00235-b. Neuroscience. 1995. PMID: 8637620
Cited by
-
Protective effect of omega-3 polyunsaturated fatty acids on sepsis via the AMPK/mTOR pathway.Pharm Biol. 2023 Dec;61(1):306-315. doi: 10.1080/13880209.2023.2168018. Pharm Biol. 2023. PMID: 36694426 Free PMC article.
-
Gα14 subunit-mediated inhibition of voltage-gated Ca2+ and K+ channels via neurokinin-1 receptors in rat celiac-superior mesenteric ganglion neurons.J Neurophysiol. 2016 Mar;115(3):1577-86. doi: 10.1152/jn.00980.2015. Epub 2016 Feb 3. J Neurophysiol. 2016. PMID: 26843606 Free PMC article.
References
-
- Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. (2006) Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34: 344–353. - PubMed
-
- Buras JA, Holzmann B, Sitkovsky M (2005) Animal models of sepsis: setting the stage. Nat Rev Drug Discov 4: 854–865. - PubMed
-
- Landry DW, Oliver JA (2001) The pathogenesis of vasodilatory shock. N Engl J Med 345: 588–595. - PubMed
-
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES (2000) The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 52: 595–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

