The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase
- PMID: 26017313
- PMCID: PMC4961239
- DOI: 10.1038/nature14492
The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase
Retraction in
-
Retraction Note: The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase.Nature. 2023 May;617(7959):208. doi: 10.1038/s41586-023-06048-x. Nature. 2023. PMID: 37069265 Free PMC article. No abstract available.
Abstract
Tumour metastasis is a complex process involving reciprocal interplay between cancer cells and host stroma at both primary and secondary sites, and is strongly influenced by microenvironmental factors such as hypoxia. Tumour-secreted proteins play a crucial role in these interactions and present strategic therapeutic potential. Metastasis of breast cancer to the bone affects approximately 85% of patients with advanced disease and renders them largely untreatable. Specifically, osteolytic bone lesions, where bone is destroyed, lead to debilitating skeletal complications and increased patient morbidity and mortality. The molecular interactions governing the early events of osteolytic lesion formation are currently unclear. Here we show hypoxia to be specifically associated with bone relapse in patients with oestrogen-receptor negative breast cancer. Global quantitative analysis of the hypoxic secretome identified lysyl oxidase (LOX) as significantly associated with bone-tropism and relapse. High expression of LOX in primary breast tumours or systemic delivery of LOX leads to osteolytic lesion formation whereas silencing or inhibition of LOX activity abrogates tumour-driven osteolytic lesion formation. We identify LOX as a novel regulator of NFATc1-driven osteoclastogenesis, independent of RANK ligand, which disrupts normal bone homeostasis leading to the formation of focal pre-metastatic lesions. We show that these lesions subsequently provide a platform for circulating tumour cells to colonize and form bone metastases. Our study identifies a novel mechanism of regulation of bone homeostasis and metastasis, opening up opportunities for novel therapeutic intervention with important clinical implications.
Conflict of interest statement
Figures
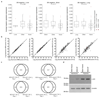

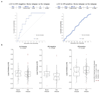

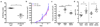

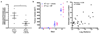




Comment in
-
Cancer: Opening LOX to metastasis.Nature. 2015 Jun 4;522(7554):41-2. doi: 10.1038/nature14529. Epub 2015 May 27. Nature. 2015. PMID: 26017311 No abstract available.
-
Metastasis: LOX does some prepping.Nat Rev Cancer. 2015 Jul;15(7):384. doi: 10.1038/nrc3976. Epub 2015 Jun 18. Nat Rev Cancer. 2015. PMID: 26084391 No abstract available.
-
Cancer: LOX does some prepping.Nat Rev Drug Discov. 2015 Jul;14(7):458-9. doi: 10.1038/nrd4674. Nat Rev Drug Discov. 2015. PMID: 26129796 No abstract available.
Similar articles
-
Lysyl oxidase is essential for hypoxia-induced metastasis.Nature. 2006 Apr 27;440(7088):1222-6. doi: 10.1038/nature04695. Nature. 2006. Retraction in: Nature. 2020 Mar;579(7799):456. doi: 10.1038/s41586-020-2112-4 PMID: 16642001 Retracted.
-
P2Y2R activation by nucleotides released from the highly metastatic breast cancer cell MDA-MB-231 contributes to pre-metastatic niche formation by mediating lysyl oxidase secretion, collagen crosslinking, and monocyte recruitment.Oncotarget. 2014 Oct 15;5(19):9322-34. doi: 10.18632/oncotarget.2427. Oncotarget. 2014. PMID: 25238333 Free PMC article.
-
Lysyl Oxidase Is a Strong Determinant of Tumor Cell Colonization in Bone.Cancer Res. 2017 Jan 15;77(2):268-278. doi: 10.1158/0008-5472.CAN-15-2621. Epub 2016 Oct 14. Cancer Res. 2017. PMID: 27742687
-
Lysyl Oxidase, a Targetable Secreted Molecule Involved in Cancer Metastasis.Cancer Res. 2016 Jan 15;76(2):188-92. doi: 10.1158/0008-5472.CAN-15-2306. Epub 2016 Jan 5. Cancer Res. 2016. PMID: 26732355 Review.
-
Lysyl oxidase mediates hypoxic control of metastasis.Cancer Res. 2006 Nov 1;66(21):10238-41. doi: 10.1158/0008-5472.CAN-06-3197. Cancer Res. 2006. PMID: 17079439 Review.
Cited by
-
Extracellular Vesicle-Mediated Bone Remodeling and Bone Metastasis: Implications in Prostate Cancer.Subcell Biochem. 2021;97:297-361. doi: 10.1007/978-3-030-67171-6_12. Subcell Biochem. 2021. PMID: 33779922 Review.
-
Osteoclast Signal Transduction During Bone Metastasis Formation.Front Cell Dev Biol. 2020 Jun 19;8:507. doi: 10.3389/fcell.2020.00507. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32637413 Free PMC article. Review.
-
Force Matters: Biomechanical Regulation of Cell Invasion and Migration in Disease.Trends Cell Biol. 2016 Jul;26(7):486-497. doi: 10.1016/j.tcb.2016.03.007. Epub 2016 Apr 4. Trends Cell Biol. 2016. PMID: 27056543 Free PMC article. Review.
-
Cell Cytoskeleton and Stiffness Are Mechanical Indicators of Organotropism in Breast Cancer.Biology (Basel). 2021 Mar 25;10(4):259. doi: 10.3390/biology10040259. Biology (Basel). 2021. PMID: 33805866 Free PMC article.
-
Immunogenic hydrogel toolkit disturbing residual tumor "seeds" and pre-metastatic "soil" for inhibition of postoperative tumor recurrence and metastasis.Acta Pharm Sin B. 2022 Aug;12(8):3383-3397. doi: 10.1016/j.apsb.2022.02.017. Epub 2022 Feb 24. Acta Pharm Sin B. 2022. PMID: 35967277 Free PMC article.
References
-
- Chan DA, Giaccia AJ. Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev. 2007;26:333–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

