Enhancing gene delivery of adeno-associated viruses by cell-permeable peptides
- PMID: 26015948
- PMCID: PMC4365833
- DOI: 10.1038/mtm.2013.12
Enhancing gene delivery of adeno-associated viruses by cell-permeable peptides
Abstract
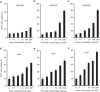
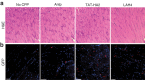
Adeno-associated virus type 2 (AAV2) is considered a promising gene delivery vector and has been extensively applied in several disease models; however, inefficient transduction in various cells and tissues has limited its widespread application in many areas of gene therapy. In this study, we have developed a general, but efficient, strategy to enhance viral transduction, both in vitro and in vivo, by incubating viral particles with cell-permeable peptides (CPPs). We show that CPPs increase internalization of viral particles into cells by facilitating both energy-independent and energy-dependent endocytosis. Moreover, CPPs can significantly enhance the endosomal escape process of viral particles, thus enhancing viral transduction to those cells that have exhibited very low permissiveness to AAV2 infection as a result of impaired intracellular viral processing. We also demonstrated that this approach could be applicable to other AAV serotypes. Thus, the membrane-penetrating ability of CPPs enables us to generate an efficient method for enhanced gene delivery of AAV vectors, potentially facilitating its applicability to human gene therapy.
Figures
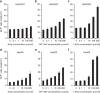
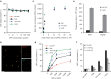
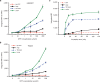
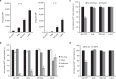
Similar articles
-
Transduction of Adeno-Associated Virus Vectors Targeting Hair Cells and Supporting Cells in the Neonatal Mouse Cochlea.Front Cell Neurosci. 2019 Jan 24;13:8. doi: 10.3389/fncel.2019.00008. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30733670 Free PMC article.
-
Identification and Validation of Small Molecules That Enhance Recombinant Adeno-associated Virus Transduction following High-Throughput Screens.J Virol. 2016 Jul 27;90(16):7019-7031. doi: 10.1128/JVI.02953-15. Print 2016 Aug 15. J Virol. 2016. PMID: 27147738 Free PMC article.
-
Insertional mutagenesis of the adeno-associated virus type 2 (AAV2) capsid gene and generation of AAV2 vectors targeted to alternative cell-surface receptors.Hum Gene Ther. 2001 Sep 20;12(14):1697-711. doi: 10.1089/104303401750476212. Hum Gene Ther. 2001. PMID: 11560765
-
Intracellular Delivery of Molecular Cargo Using Cell-Penetrating Peptides and the Combination Strategies.Int J Mol Sci. 2015 Aug 18;16(8):19518-36. doi: 10.3390/ijms160819518. Int J Mol Sci. 2015. PMID: 26295227 Free PMC article. Review.
-
Designer gene delivery vectors: molecular engineering and evolution of adeno-associated viral vectors for enhanced gene transfer.Pharm Res. 2008 Mar;25(3):489-99. doi: 10.1007/s11095-007-9431-0. Epub 2007 Sep 1. Pharm Res. 2008. PMID: 17763830 Free PMC article. Review.
Cited by
-
Emerging Technologies for Delivery of Biotherapeutics and Gene Therapy Across the Blood-Brain Barrier.BioDrugs. 2018 Dec;32(6):547-559. doi: 10.1007/s40259-018-0309-y. BioDrugs. 2018. PMID: 30306341 Free PMC article.
-
Adhesive thermosensitive gels for local delivery of viral vectors.Biotechnol Bioeng. 2019 Sep;116(9):2353-2363. doi: 10.1002/bit.27007. Epub 2019 May 20. Biotechnol Bioeng. 2019. PMID: 31038193 Free PMC article.
-
Charged group surface accessibility determines micelleplexes formation and cellular interaction.Nanoscale. 2015 May 7;7(17):7559-64. doi: 10.1039/c5nr00095e. Nanoscale. 2015. PMID: 25866141 Free PMC article.
-
CRISPR Modeling and Correction of Cardiovascular Disease.Circ Res. 2022 Jun 10;130(12):1827-1850. doi: 10.1161/CIRCRESAHA.122.320496. Epub 2022 Jun 9. Circ Res. 2022. PMID: 35679361 Free PMC article. Review.
-
Lipid interactions of LAH4, a peptide with antimicrobial and nucleic acid transfection activities.Eur Biophys J. 2014 Nov;43(10-11):499-507. doi: 10.1007/s00249-014-0980-y. Epub 2014 Sep 3. Eur Biophys J. 2014. PMID: 25182242
References
-
- Fisher KJ, Jooss K, Alston J, Yang Y, Haecker SE, High K. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. - PubMed
-
- Mueller C, Flotte TR. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15:858–863. - PubMed
-
- Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–327. - PubMed
-
- Girod A, Ried M, Wobus C, Lahm H, Leike K, Kleinschmidt J. Genetic capsid modifications allow efficient re-targeting of adeno-associated virus type 2. Nat Med. 1999;5:1052–1056. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

