Effects of Local Heart Irradiation in a Glutathione S-Transferase Alpha 4-Null Mouse Model
- PMID: 26010708
- PMCID: PMC4688037
- DOI: 10.1667/RR13979.1
Effects of Local Heart Irradiation in a Glutathione S-Transferase Alpha 4-Null Mouse Model
Abstract
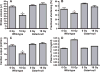
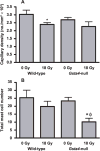
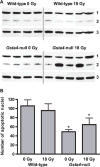
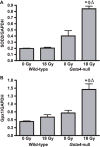
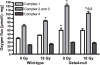
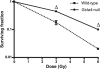
Glutathione S-transferase alpha 4 (GSTA4-4) is one of the enzymes responsible for the removal of 4-hydroxynonenal (4-HNE), an electrophilic product of lipid peroxidation in cellular membranes during oxidative stress. 4-HNE is a direct activator of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), a transcription factor with many target genes encoding antioxidant and anti-electrophile enzymes. We have previously shown that Gsta4-null mice on a 129/Sv background exhibited increased activity of Nrf2 in the heart. Here we examined the sensitivity of this Gsta4-null mouse model towards cardiac function and structure loss due to local heart irradiation. Male Gsta4-null and wild-type mice were exposed to a single X-ray dose of 18 Gy to the heart. Six months after irradiation, immunohistochemical staining for respiratory complexes 2 and 5 indicated that radiation exposure had caused most pronounced alterations in mitochondrial morphology in Gsta4-null mice. On the other hand, wild-type mice showed a decline in cardiac function and an increase in plasma levels of troponin-I, while no such changes were observed in Gsta4-null mice. Radiation-induced Nrf2-target gene expression only in Gsta4-null mice. In conclusion, although loss of GSTA4-4 led to enhanced susceptibility of cardiac mitochondria to radiation-induced loss of morphology, cardiac function was preserved in Gsta4-null mice. We propose that this protection against cardiac function loss may occur, at least in part, by upregulation of the Nrf2 pathway.
Figures

Similar articles
-
Protection from oxidative and electrophilic stress in the Gsta4-null mouse heart.Cardiovasc Toxicol. 2013 Dec;13(4):347-56. doi: 10.1007/s12012-013-9215-1. Cardiovasc Toxicol. 2013. PMID: 23690225 Free PMC article.
-
Gsta4 Null Mouse Embryonic Fibroblasts Exhibit Enhanced Sensitivity to Oxidants: Role of 4-Hydroxynonenal in Oxidant Toxicity.Open J Apoptosis. 2013 Jan 1;2(1):10.4236/ojapo.2013.21001. doi: 10.4236/ojapo.2013.21001. Open J Apoptosis. 2013. PMID: 24353929 Free PMC article.
-
Knockout of the Gsta4 Gene in Male Mice Leads to an Altered Pattern of Hepatic Protein Carbonylation and Enhanced Inflammation Following Chronic Consumption of an Ethanol Diet.Alcohol Clin Exp Res. 2018 Jul;42(7):1192-1205. doi: 10.1111/acer.13766. Epub 2018 May 30. Alcohol Clin Exp Res. 2018. PMID: 29708596 Free PMC article.
-
Nrf2 and antioxidant defense against CYP2E1 toxicity.Expert Opin Drug Metab Toxicol. 2009 Oct;5(10):1223-44. doi: 10.1517/17425250903143769. Expert Opin Drug Metab Toxicol. 2009. PMID: 19671018 Review.
-
Dual localization of glutathione S-transferase in the cytosol and mitochondria: implications in oxidative stress, toxicity and disease.FEBS J. 2011 Nov;278(22):4243-51. doi: 10.1111/j.1742-4658.2011.08358.x. Epub 2011 Oct 12. FEBS J. 2011. PMID: 21929724 Free PMC article. Review.
Cited by
-
Radiation-induced heart disease: a review of classification, mechanism and prevention.Int J Biol Sci. 2019 Aug 8;15(10):2128-2138. doi: 10.7150/ijbs.35460. eCollection 2019. Int J Biol Sci. 2019. PMID: 31592122 Free PMC article. Review.
-
GSTA4 mediates reduction of cisplatin ototoxicity in female mice.Nat Commun. 2019 Sep 12;10(1):4150. doi: 10.1038/s41467-019-12073-0. Nat Commun. 2019. PMID: 31515474 Free PMC article.
-
Effects of ionizing radiation on the heart.Mutat Res Rev Mutat Res. 2016 Oct-Dec;770(Pt B):319-327. doi: 10.1016/j.mrrev.2016.07.003. Epub 2016 Jul 10. Mutat Res Rev Mutat Res. 2016. PMID: 27919338 Free PMC article. Review.
-
Characterization of cardiovascular injury in mice following partial-heart irradiation with clinically relevant dose and fractionation.Radiother Oncol. 2021 Apr;157:155-162. doi: 10.1016/j.radonc.2021.01.023. Epub 2021 Feb 3. Radiother Oncol. 2021. PMID: 33545252 Free PMC article.
-
Late Effects of Heavy-Ion Space Radiation on Splenocyte Subpopulations and NK Cytotoxic Function.Front Astron Space Sci. 2022;9:949432. doi: 10.3389/fspas.2022.949432. Epub 2022 Nov 7. Front Astron Space Sci. 2022. PMID: 39554816 Free PMC article.
References
-
- Engle MR, Singh SP, Czernik PJ, Gaddy D, Montague DC, Ceci JD, et al. Physiological role of mGSTA4-4, a glutathione S-transferase metabolizing 4-hydroxynonenal: generation and analysis of mGsta4 null mouse. Toxicol Appl Pharmacol. 2004;194:296–308. - PubMed
-
- Singh SP, Niemczyk M, Saini D, Awasthi YC, Zimniak L, Zimniak P. Role of the electrophilic lipid peroxidation product 4-hydroxynonenal in the development and maintenance of obesity in mice. Biochemistry. 2008;47:3900–11. - PubMed
-
- Chen ZH, Saito Y, Yoshida Y, Sekine A, Noguchi N, Niki E. 4-Hydroxynonenal induces adaptive response and enhances PC12 cell tolerance primarily through induction of thioredoxin reductase 1 via activation of Nrf2. J Biol Chem. 2005;280:41921–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

