Identification of Non-HIV Immunogens That Bind to Germline b12 Predecessors and Prime for Elicitation of Cross-clade Neutralizing HIV-1 Antibodies
- PMID: 26010511
- PMCID: PMC4444298
- DOI: 10.1371/journal.pone.0126428
Identification of Non-HIV Immunogens That Bind to Germline b12 Predecessors and Prime for Elicitation of Cross-clade Neutralizing HIV-1 Antibodies
Abstract
A fundamental challenge for developing an effective and safe HIV-1 vaccine is to identify vaccine immunogens that can initiate and maintain immune responses leading to elicitation of broadly neutralizing HIV-1 antibodies (bnAbs) through complex maturation pathways. We have previously found that HIV-1 envelope glycoproteins (Env) lack measurable binding to putative germline predecessors of known bnAbs and proposed to search for non-HIV immunogens that could initiate their somatic maturation. Using bnAb b12 as a model bnAb and yeast display technology, we isolated five (poly)peptides from plant leaves, insects, E. coli strains, and sea water microbes that bind to b12 putative germline and intermediate antibodies. Rabbit immunization with the (poly)peptides alone induced high titers of cross-reactive antibodies that neutralized HIV-1 isolates SF162 and JRFL. Priming rabbits with the (poly)peptides followed by boosts with trimeric gp140SF162 and then resurfaced Env (RSC3) induced antibodies that competed with mature b12 and neutralized tier 1 and 2 viruses from clade B, C and E, while control rabbits without (poly)peptide priming induced antibodies that did not compete with mature b12 and neutralized fewer isolates. The degree of competition with mature b12 for binding to gp140SF162 correlated with the neutralizing activity of the rabbit IgG. Reversing the order of the two boosting immunogens significantly affected the binding profile and neutralization potency of the rabbit IgG. Our study is the first to provide evidence that appears to support the concept that non-HIV immunogens may initiate immune responses leading to elicitation of cross-clade neutralizing antibodies.
Conflict of interest statement
Figures
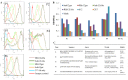
Similar articles
-
A Trimeric HIV-1 Envelope gp120 Immunogen Induces Potent and Broad Anti-V1V2 Loop Antibodies against HIV-1 in Rabbits and Rhesus Macaques.J Virol. 2018 Feb 12;92(5):e01796-17. doi: 10.1128/JVI.01796-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237847 Free PMC article.
-
Putative rhesus macaque germline predecessors of human broadly HIV-neutralizing antibodies: differences from the human counterparts and implications for HIV-1 vaccine development.Vaccine. 2011 Sep 16;29(40):6903-10. doi: 10.1016/j.vaccine.2011.07.046. Epub 2011 Jul 30. Vaccine. 2011. PMID: 21807049 Free PMC article.
-
A single mutation turns a non-binding germline-like predecessor of broadly neutralizing antibody into a binding antibody to HIV-1 envelope glycoproteins.MAbs. 2011 Jul-Aug;3(4):402-7. doi: 10.4161/mabs.3.4.15740. Epub 2011 Jul 1. MAbs. 2011. PMID: 21540646 Free PMC article.
-
Strategies for induction of HIV-1 envelope-reactive broadly neutralizing antibodies.J Int AIDS Soc. 2021 Nov;24 Suppl 7(Suppl 7):e25831. doi: 10.1002/jia2.25831. J Int AIDS Soc. 2021. PMID: 34806332 Free PMC article. Review.
-
Targeting broadly neutralizing antibody precursors: a naïve approach to vaccine design.Curr Opin HIV AIDS. 2019 Jul;14(4):294-301. doi: 10.1097/COH.0000000000000548. Curr Opin HIV AIDS. 2019. PMID: 30946041 Review.
Cited by
-
Epitope-focused immunogens against the CD4-binding site of HIV-1 envelope protein induce neutralizing antibodies against auto- and heterologous viruses.J Biol Chem. 2018 Jan 19;293(3):830-846. doi: 10.1074/jbc.M117.816447. Epub 2017 Nov 29. J Biol Chem. 2018. PMID: 29187598 Free PMC article.
-
Brief introduction of current technologies in isolation of broadly neutralizing HIV-1 antibodies.Virus Res. 2018 Jan 2;243:75-82. doi: 10.1016/j.virusres.2017.10.011. Epub 2017 Oct 16. Virus Res. 2018. PMID: 29051051 Free PMC article. Review.
-
The Significance of a Common Idiotype (1F7) on Antibodies against Human Immune Deficiency Virus Type 1 and Hepatitis C Virus.Front Oncol. 2016 Feb 5;6:11. doi: 10.3389/fonc.2016.00011. eCollection 2016. Front Oncol. 2016. PMID: 26904499 Free PMC article. Review.
-
Identification of a HIV Gp41-Specific Human Monoclonal Antibody With Potent Antibody-Dependent Cellular Cytotoxicity.Front Immunol. 2018 Nov 16;9:2613. doi: 10.3389/fimmu.2018.02613. eCollection 2018. Front Immunol. 2018. PMID: 30519238 Free PMC article.
-
The Antibody Germline/Maturation Hypothesis, Elicitation of Broadly Neutralizing Antibodies Against HIV-1 and Cord Blood IgM Repertoires.Front Immunol. 2014 Aug 28;5:398. doi: 10.3389/fimmu.2014.00398. eCollection 2014. Front Immunol. 2014. PMID: 25221552 Free PMC article.
References
-
- Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PW, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 1994,266:1024–1027. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

