DECIDER: prospective randomized multicenter phase II trial of low-dose decitabine (DAC) administered alone or in combination with the histone deacetylase inhibitor valproic acid (VPA) and all-trans retinoic acid (ATRA) in patients >60 years with acute myeloid leukemia who are ineligible for induction chemotherapy
- PMID: 26008690
- PMCID: PMC4443550
- DOI: 10.1186/s12885-015-1432-5
DECIDER: prospective randomized multicenter phase II trial of low-dose decitabine (DAC) administered alone or in combination with the histone deacetylase inhibitor valproic acid (VPA) and all-trans retinoic acid (ATRA) in patients >60 years with acute myeloid leukemia who are ineligible for induction chemotherapy
Abstract
Background: Acute myeloid leukemia (AML) is predominantly a disease of older patients with a poor long-term survival. Approval of decitabine (DAC) in the European Union (EU) in 2012 for the treatment of patients with AML ≥65 years marks the potential for hypomethylating agents in elderly AML. Nevertheless the situation is dissatisfactory and the quest for novel treatment approaches, including combination epigenetic therapy is actively ongoing. The given randomized trial should be helpful in investigating the question whether combinations of DAC with the histone deacetylase (HDAC) inhibitor valproic acid (VPA) and/or all-trans retinoic acid (ATRA), which in vitro show a very promising synergism, are superior to the DAC monotherapy. The accompanying translational research project will contribute to find surrogate molecular end points for drug efficacy and better tailor epigenetic therapy. An additional aim of the study is to investigate the prognostic value of geriatric assessments for elderly AML patients treated non-intensively.
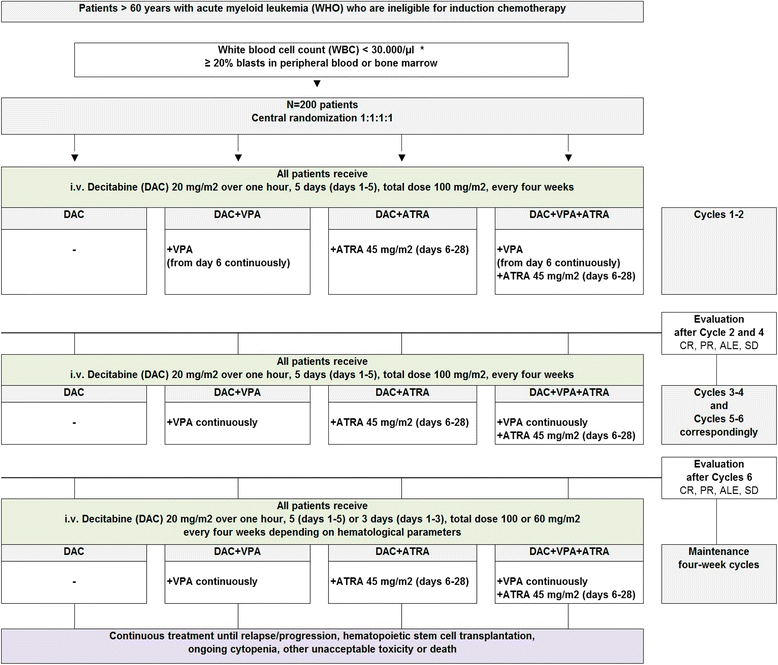
Methods/design: DECIDER is a prospective, randomized, observer blind, parallel group, multicenter, Phase II study with a 2x2 factorial design. The primary endpoint is objective best overall response (complete remission (CR) and partial remission (PR)). The target population is AML patients aged 60 years or older and unfit for standard induction chemotherapy. Patients are randomized to one of the four treatment groups: DAC alone or in combination with VPA or ATRA or with both add-on drugs. One interim safety analysis was planned and carried out with the objective to stop early one or more of the treatment arms in case of an unacceptable death rate. This analysis showed that in all treatment arms the critical stopping rule was not reached. No important safety issues were observed. The Data Monitoring Committee (DMC) recommended continuing the study as planned. The first patient was included in December 2011. A total of 189 out of 200 planned patients were randomized since then (status 31.12.2014).
Trial registration: ClinicalTrials.gov identifier: NCT00867672 (registration date 23.03.2009); German clinical trials registry number: DRKS00000733 (registration date 19.04.2011).
Figures
Similar articles
-
Valproate and Retinoic Acid in Combination With Decitabine in Elderly Nonfit Patients With Acute Myeloid Leukemia: Results of a Multicenter, Randomized, 2 × 2, Phase II Trial.J Clin Oncol. 2020 Jan 20;38(3):257-270. doi: 10.1200/JCO.19.01053. Epub 2019 Dec 3. J Clin Oncol. 2020. PMID: 31794324 Clinical Trial.
-
Clinical trial of valproic acid and all-trans retinoic acid in patients with poor-risk acute myeloid leukemia.Cancer. 2005 Dec 15;104(12):2717-25. doi: 10.1002/cncr.21589. Cancer. 2005. PMID: 16294345 Clinical Trial.
-
Results of phase 2 randomized study of low-dose decitabine with or without valproic acid in patients with myelodysplastic syndrome and acute myelogenous leukemia.Cancer. 2015 Feb 15;121(4):556-61. doi: 10.1002/cncr.29085. Epub 2014 Oct 21. Cancer. 2015. PMID: 25336333 Free PMC article. Clinical Trial.
-
Conventional chemotherapy or hypomethylating agents for older patients with acute myeloid leukaemia?Hematol Oncol. 2014 Mar;32(1):1-9. doi: 10.1002/hon.2046. Epub 2013 Mar 20. Hematol Oncol. 2014. PMID: 23512815 Review.
-
Valproic acid and all-trans retinoic acid: meta-analysis of a palliative treatment regimen in AML and MDS patients.Onkologie. 2008 Nov;31(11):629-33. doi: 10.1159/000160599. Epub 2008 Oct 23. Onkologie. 2008. PMID: 19145098 Review.
Cited by
-
Geriatric assessment for older adults receiving less-intensive therapy for acute myeloid leukemia: report of CALGB 361101.Blood Adv. 2022 Jun 28;6(12):3812-3820. doi: 10.1182/bloodadvances.2021006872. Blood Adv. 2022. PMID: 35420672 Free PMC article. Clinical Trial.
-
Combined epigenetic and differentiation-based treatment inhibits neuroblastoma tumor growth and links HIF2α to tumor suppression.Proc Natl Acad Sci U S A. 2017 Jul 25;114(30):E6137-E6146. doi: 10.1073/pnas.1700655114. Epub 2017 Jul 10. Proc Natl Acad Sci U S A. 2017. PMID: 28696319 Free PMC article.
-
Molecular dissection of valproic acid effects in acute myeloid leukemia identifies predictive networks.Epigenetics. 2016 Jul 2;11(7):517-25. doi: 10.1080/15592294.2016.1187350. Epub 2016 Jun 16. Epigenetics. 2016. PMID: 27309669 Free PMC article. Clinical Trial.
-
Efficacy and safety of decitabine in treatment of elderly patients with acute myeloid leukemia: A systematic review and meta-analysis.Oncotarget. 2017 Jun 20;8(25):41498-41507. doi: 10.18632/oncotarget.17241. Oncotarget. 2017. PMID: 28489568 Free PMC article. Review.
-
Nanoacetylated N-(4-Hydroxyphenyl) Retinamide Modulates Histone Acetylation-Methylation Epigenetic Disparity to Restrict Epithelial-Mesenchymal Transition in Neuroblastoma.ACS Med Chem Lett. 2022 Jun 27;13(7):1109-1117. doi: 10.1021/acsmedchemlett.2c00135. eCollection 2022 Jul 14. ACS Med Chem Lett. 2022. PMID: 35859882 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials