Polo-like kinase 2 regulates angiogenic sprouting and blood vessel development
- PMID: 26004360
- PMCID: PMC4515213
- DOI: 10.1016/j.ydbio.2015.05.011
Polo-like kinase 2 regulates angiogenic sprouting and blood vessel development
Abstract
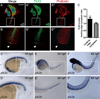
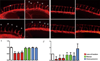
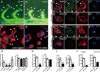
Angiogenesis relies on specialized endothelial tip cells to extend toward guidance cues in order to direct growing blood vessels. Although many of the signaling pathways that control this directional endothelial sprouting are well known, the specific cellular mechanisms that mediate this process remain to be fully elucidated. Here, we show that Polo-like kinase 2 (PLK2) regulates Rap1 activity to guide endothelial tip cell lamellipodia formation and subsequent angiogenic sprouting. Using a combination of high-resolution in vivo imaging of zebrafish vascular development and a human umbilical vein endothelial cell (HUVEC) in vitro cell culture system, we observed that loss of PLK2 function resulted in a reduction in endothelial cell sprouting and migration, whereas overexpression of PLK2 promoted angiogenesis. Furthermore, we discovered that PLK2 may control angiogenic sprouting by binding to PDZ-GEF to regulate RAP1 activity during endothelial cell lamellipodia formation and extracellular matrix attachment. Consistent with these findings, constitutively active RAP1 could rescue the endothelial cell sprouting defects observed in zebrafish and HUVEC PLK2 knockdowns. Overall, these findings reveal a conserved PLK2-RAP1 pathway that is crucial to regulate endothelial tip cell behavior in order to ensure proper vascular development and patterning in vertebrates.
Keywords: Angiogenesis; Human umbilical vein endothelial cells; Polo-like kinase 2; Vascular development; Zebrafish.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
The neuronal transcription factor NPAS4 is a strong inducer of sprouting angiogenesis and tip cell formation.Cardiovasc Res. 2017 Feb;113(2):222-223. doi: 10.1093/cvr/cvw248. Epub 2017 Jan 12. Cardiovasc Res. 2017. PMID: 28082451
-
Role of afadin in vascular endothelial growth factor- and sphingosine 1-phosphate-induced angiogenesis.Circ Res. 2010 Jun 11;106(11):1731-42. doi: 10.1161/CIRCRESAHA.110.216747. Epub 2010 Apr 22. Circ Res. 2010. PMID: 20413783
-
FGD5 Regulates VEGF Receptor-2 Coupling to PI3 Kinase and Receptor Recycling.Arterioscler Thromb Vasc Biol. 2017 Dec;37(12):2301-2310. doi: 10.1161/ATVBAHA.117.309978. Epub 2017 Oct 19. Arterioscler Thromb Vasc Biol. 2017. PMID: 29051140
-
Regulation of angiogenesis by a small GTPase Rap1.Vascul Pharmacol. 2010 Jul-Aug;53(1-2):1-10. doi: 10.1016/j.vph.2010.03.003. Epub 2010 Mar 16. Vascul Pharmacol. 2010. PMID: 20302970 Review.
-
Distinct functions for Rap1 signaling in vascular morphogenesis and dysfunction.Exp Cell Res. 2013 Sep 10;319(15):2350-9. doi: 10.1016/j.yexcr.2013.07.022. Epub 2013 Aug 2. Exp Cell Res. 2013. PMID: 23911990 Free PMC article. Review.
Cited by
-
Cytokinetic bridge triggers de novo lumen formation in vivo.Nat Commun. 2020 Mar 9;11(1):1269. doi: 10.1038/s41467-020-15002-8. Nat Commun. 2020. PMID: 32152267 Free PMC article.
-
Expansion of macrophage and liver sinusoidal endothelial cell subpopulations during non-alcoholic steatohepatitis progression.iScience. 2023 Apr 5;26(5):106572. doi: 10.1016/j.isci.2023.106572. eCollection 2023 May 19. iScience. 2023. PMID: 37124414 Free PMC article.
-
Esm-1 mediates transcriptional polarization associated with diabetic kidney disease.Am J Physiol Renal Physiol. 2024 Jun 1;326(6):F1016-F1031. doi: 10.1152/ajprenal.00419.2023. Epub 2024 Apr 11. Am J Physiol Renal Physiol. 2024. PMID: 38601985
-
The pyrazolyl-urea GeGe3 inhibits tumor angiogenesis and reveals dystrophia myotonica protein kinase (DMPK)1 as a novel angiogenesis target.Oncotarget. 2017 Nov 21;8(64):108195-108212. doi: 10.18632/oncotarget.22598. eCollection 2017 Dec 8. Oncotarget. 2017. PMID: 29296234 Free PMC article.
-
New Gene Markers for Metabolic Processes and Homeostasis in Porcine Buccal Pouch Mucosa during Cells Long Term-Cultivation-A Primary Culture Approach.Int J Mol Sci. 2018 Mar 29;19(4):1027. doi: 10.3390/ijms19041027. Int J Mol Sci. 2018. PMID: 29596348 Free PMC article.
References
-
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nature reviews. Molecular cell biology. 2007;8:464–478. - PubMed
-
- Bentley D, Toroian-Raymond A. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 1986;323:712–715. - PubMed
-
- Bivona TG, Quatela S, Philips MR. Analysis of Ras activation in living cells with GFP-RBD. Methods in enzymology. 2006;407:128–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

