The Cell-Intrinsic Circadian Clock Is Dispensable for Lymphocyte Differentiation and Function
- PMID: 26004187
- PMCID: PMC4464971
- DOI: 10.1016/j.celrep.2015.04.058
The Cell-Intrinsic Circadian Clock Is Dispensable for Lymphocyte Differentiation and Function
Abstract
Circadian rhythms regulate many aspects of physiology, ranging from sleep-wake cycles and metabolic parameters to susceptibility to infection. The molecular clock, with transcription factor BMAL1 at its core, controls both central and cell-intrinsic circadian rhythms. Using a circadian reporter, we observed dynamic regulation of clock activity in lymphocytes. However, its disruption upon conditional Bmal1 ablation did not alter T- or B-cell differentiation or function. Although the magnitude of interleukin 2 (IL-2) production was affected by the time of bacterial infection, it was independent of cell-intrinsic expression of BMAL1. The circadian gating of the IL-2 response was preserved in Bmal1-deficient T cells, despite a slight reduction in cytokine production in a competitive setting. Our results suggest that, contrary to the prevailing view, the adaptive immune response is not affected by the cell-intrinsic clock but is likely influenced by cell-extrinsic circadian cues operating across multiple cell types.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
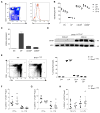



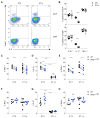
Similar articles
-
Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival.Sci Transl Med. 2016 Feb 3;8(324):324ra16. doi: 10.1126/scitranslmed.aad3305. Epub 2016 Feb 3. Sci Transl Med. 2016. PMID: 26843191 Free PMC article.
-
Deficiency of circadian clock protein BMAL1 in mice results in a low bone mass phenotype.Bone. 2016 Mar;84:194-203. doi: 10.1016/j.bone.2016.01.006. Epub 2016 Jan 14. Bone. 2016. PMID: 26789548 Free PMC article.
-
Tissue-intrinsic dysfunction of circadian clock confers transplant arteriosclerosis.Proc Natl Acad Sci U S A. 2011 Oct 11;108(41):17147-52. doi: 10.1073/pnas.1112998108. Epub 2011 Oct 3. Proc Natl Acad Sci U S A. 2011. PMID: 21969583 Free PMC article.
-
The functional significance of the skeletal muscle clock: lessons from Bmal1 knockout models.Skelet Muscle. 2016 Oct 13;6:33. doi: 10.1186/s13395-016-0107-5. eCollection 2016. Skelet Muscle. 2016. PMID: 27752300 Free PMC article. Review.
-
Circadian modification network of a core clock driver BMAL1 to harmonize physiology from brain to peripheral tissues.Neurochem Int. 2018 Oct;119:11-16. doi: 10.1016/j.neuint.2017.12.013. Epub 2018 Jan 3. Neurochem Int. 2018. PMID: 29305918 Review.
Cited by
-
Diurnal Rhythmicity Programs of Microbiota and Transcriptional Oscillation of Circadian Regulator, NFIL3.Front Immunol. 2020 Sep 10;11:552188. doi: 10.3389/fimmu.2020.552188. eCollection 2020. Front Immunol. 2020. PMID: 33013924 Free PMC article. Review.
-
Nutrients and the microenvironment to feed a T cell army.Semin Immunol. 2016 Oct;28(5):505-513. doi: 10.1016/j.smim.2016.09.003. Epub 2016 Oct 3. Semin Immunol. 2016. PMID: 27712958 Free PMC article. Review.
-
Metabolic Adaptations of CD4+ T Cells in Inflammatory Disease.Front Immunol. 2018 Mar 15;9:540. doi: 10.3389/fimmu.2018.00540. eCollection 2018. Front Immunol. 2018. PMID: 29599783 Free PMC article. Review.
-
Association of Circadian Clock Gene Expression with Pediatric/Adolescent Asthma and Its Comorbidities.Int J Mol Sci. 2023 Apr 19;24(8):7477. doi: 10.3390/ijms24087477. Int J Mol Sci. 2023. PMID: 37108640 Free PMC article.
-
Circadian Clock Genes Are Correlated with Prognosis and Immune Cell Infiltration in Colon Adenocarcinoma.Comput Math Methods Med. 2022 Jan 25;2022:1709918. doi: 10.1155/2022/1709918. eCollection 2022. Comput Math Methods Med. 2022. PMID: 35116071 Free PMC article.
References
-
- Bass J. Circadian topology of metabolism. Nature. 2012;491:348–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

