RUNX3 Controls a Metastatic Switch in Pancreatic Ductal Adenocarcinoma
- PMID: 26004068
- PMCID: PMC4458240
- DOI: 10.1016/j.cell.2015.04.048
RUNX3 Controls a Metastatic Switch in Pancreatic Ductal Adenocarcinoma
Abstract
For the majority of patients with pancreas cancer, the high metastatic proclivity is life limiting. Some patients, however, present with and succumb to locally destructive disease. A molecular understanding of these distinct disease manifestations can critically inform patient management. Using genetically engineered mouse models, we show that heterozygous mutation of Dpc4/Smad4 attenuates the metastatic potential of Kras(G12D/+);Trp53(R172H/+) pancreatic ductal adenocarcinomas while increasing their proliferation. Subsequent loss of heterozygosity of Dpc4 restores metastatic competency while further unleashing proliferation, creating a highly lethal combination. Expression levels of Runx3 respond to and combine with Dpc4 status to coordinately regulate the balance between cancer cell division and dissemination. Thus, Runx3 serves as both a tumor suppressor and promoter in slowing proliferation while orchestrating a metastatic program to stimulate cell migration, invasion, and secretion of proteins that favor distant colonization. These findings suggest a model to anticipate likely disease behaviors in patients and tailor treatment strategies accordingly.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
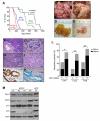
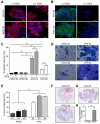
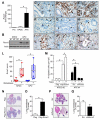
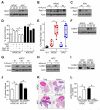
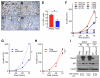
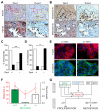
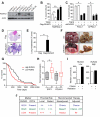
Comment in
-
It's a SMAD/SMAD World.Cell. 2015 Jun 4;161(6):1245-6. doi: 10.1016/j.cell.2015.05.030. Cell. 2015. PMID: 26046433
Similar articles
-
It's a SMAD/SMAD World.Cell. 2015 Jun 4;161(6):1245-6. doi: 10.1016/j.cell.2015.05.030. Cell. 2015. PMID: 26046433
-
RUNX3 Modulates Metastatic Potential in Pancreatic Ductal Adenocarcinoma.Cancer Discov. 2015 Jul;5(7):OF27. doi: 10.1158/2159-8290.CD-RW2015-103. Epub 2015 Jun 4. Cancer Discov. 2015. PMID: 26045017
-
SETDB1 Inhibits p53-Mediated Apoptosis and Is Required for Formation of Pancreatic Ductal Adenocarcinomas in Mice.Gastroenterology. 2020 Aug;159(2):682-696.e13. doi: 10.1053/j.gastro.2020.04.047. Epub 2020 Apr 28. Gastroenterology. 2020. PMID: 32360551
-
The Roles of Frequently Mutated Genes of Pancreatic Cancer in Regulation of Tumor Microenvironment.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820920969. doi: 10.1177/1533033820920969. Technol Cancer Res Treat. 2020. PMID: 32372692 Free PMC article. Review.
-
Genetics and Biology of Pancreatic Ductal Adenocarcinoma.Hematol Oncol Clin North Am. 2015 Aug;29(4):595-608. doi: 10.1016/j.hoc.2015.04.003. Epub 2015 Jun 10. Hematol Oncol Clin North Am. 2015. PMID: 26226899 Free PMC article. Review.
Cited by
-
Flaming the fight against cancer cells: the role of microRNA-93.Cancer Cell Int. 2020 Jun 29;20:277. doi: 10.1186/s12935-020-01349-x. eCollection 2020. Cancer Cell Int. 2020. PMID: 32612456 Free PMC article. Review.
-
HDAC2 Facilitates Pancreatic Cancer Metastasis.Cancer Res. 2022 Feb 15;82(4):695-707. doi: 10.1158/0008-5472.CAN-20-3209. Cancer Res. 2022. PMID: 34903606 Free PMC article.
-
MiR-24-3p Inhibits the Progression of Pancreatic Ductal Adenocarcinoma Through LAMB3 Downregulation.Front Oncol. 2020 Jan 21;9:1499. doi: 10.3389/fonc.2019.01499. eCollection 2019. Front Oncol. 2020. PMID: 32039003 Free PMC article.
-
KLF7 promotes pancreatic cancer growth and metastasis by up-regulating ISG expression and maintaining Golgi complex integrity.Proc Natl Acad Sci U S A. 2020 Jun 2;117(22):12341-12351. doi: 10.1073/pnas.2005156117. Epub 2020 May 19. Proc Natl Acad Sci U S A. 2020. PMID: 32430335 Free PMC article.
-
GEMMs as preclinical models for testing pancreatic cancer therapies.Dis Model Mech. 2015 Oct 1;8(10):1185-200. doi: 10.1242/dmm.021055. Dis Model Mech. 2015. PMID: 26438692 Free PMC article. Review.
References
-
- Allison DC, Piantadosi S, Hruban RH, Dooley WC, Fishman EK, Yeo CJ, Lillemoe KD, Pitt HA, Lin P, Cameron JL. DNA content and other factors associated with ten-year survival after resection of pancreatic carcinoma. J Surg Oncol. 1998;67:151–159. - PubMed
-
- Armstrong T, Packham G, Murphy LB, Bateman AC, Conti JA, Fine DR, Johnson CD, Benyon RC, Iredale JP. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:7427–7437. - PubMed
-
- Bae SC, Choi JK. Tumor suppressor activity of RUNX3. Oncogene. 2004;23:4336–4340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

