How mitochondrial dynamism orchestrates mitophagy
- PMID: 25999423
- PMCID: PMC4443843
- DOI: 10.1161/CIRCRESAHA.116.306374
How mitochondrial dynamism orchestrates mitophagy
Abstract
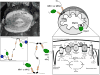
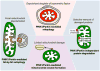
Mitochondria are highly dynamic, except in adult cardiomyocytes. Yet, the fission and fusion-promoting proteins that mediate mitochondrial dynamism are highly expressed in, and essential to the normal functioning of, hearts. Here, we review accumulating evidence supporting important roles for mitochondrial fission and fusion in cardiac mitochondrial quality control, focusing on the PTEN-induced putative kinase 1-Parkin mitophagy pathway. Based in part on recent findings from in vivo mouse models in which mitofusin-mediated mitochondrial fusion or dynamin-related protein 1-mediated mitochondrial fission was conditionally interrupted in cardiac myocytes, we propose several new concepts that may provide insight into the cardiac mitochondrial dynamism-mitophagy interactome.
Keywords: Parkinson disease; autophagy; mitochondria; mitochondrial dynamics.
© 2015 American Heart Association, Inc.
Figures

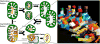
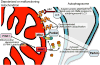
Similar articles
-
Interdependence of Parkin-Mediated Mitophagy and Mitochondrial Fission in Adult Mouse Hearts.Circ Res. 2015 Jul 31;117(4):346-51. doi: 10.1161/CIRCRESAHA.117.306859. Epub 2015 Jun 2. Circ Res. 2015. PMID: 26038571 Free PMC article.
-
Functional interplay between Parkin and Drp1 in mitochondrial fission and clearance.Biochim Biophys Acta. 2014 Sep;1843(9):2012-26. doi: 10.1016/j.bbamcr.2014.05.012. Epub 2014 May 27. Biochim Biophys Acta. 2014. PMID: 24878071
-
Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice.Science. 2015 Dec 4;350(6265):aad2459. doi: 10.1126/science.aad2459. Epub 2015 Dec 3. Science. 2015. PMID: 26785495 Free PMC article.
-
Parkin-dependent mitophagy in the heart.J Mol Cell Cardiol. 2016 Jun;95:42-9. doi: 10.1016/j.yjmcc.2015.11.023. Epub 2015 Nov 22. J Mol Cell Cardiol. 2016. PMID: 26611886 Free PMC article. Review.
-
Mitophagy and Parkinson's disease: be eaten to stay healthy.Mol Cell Neurosci. 2013 Jul;55:37-43. doi: 10.1016/j.mcn.2012.07.008. Epub 2012 Aug 2. Mol Cell Neurosci. 2013. PMID: 22926193 Review.
Cited by
-
Mitochondria Targeted Viral Replication and Survival Strategies-Prospective on SARS-CoV-2.Front Pharmacol. 2020 Aug 28;11:578599. doi: 10.3389/fphar.2020.578599. eCollection 2020. Front Pharmacol. 2020. PMID: 32982760 Free PMC article. Review.
-
Mitochondrial Surveillance by Cdc48/p97: MAD vs. Membrane Fusion.Int J Mol Sci. 2020 Sep 18;21(18):6841. doi: 10.3390/ijms21186841. Int J Mol Sci. 2020. PMID: 32961852 Free PMC article. Review.
-
Phosphatidic Acid and Cardiolipin Coordinate Mitochondrial Dynamics.Trends Cell Biol. 2018 Jan;28(1):67-76. doi: 10.1016/j.tcb.2017.08.011. Epub 2017 Sep 11. Trends Cell Biol. 2018. PMID: 28911913 Free PMC article. Review.
-
Regulation of mitochondrial bioenergetics by the non-canonical roles of mitochondrial dynamics proteins in the heart.Biochim Biophys Acta Mol Basis Dis. 2018 May;1864(5 Pt B):1991-2001. doi: 10.1016/j.bbadis.2017.09.004. Epub 2017 Sep 14. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 28918113 Free PMC article. Review.
-
MAPK1 Mediates MAM Disruption and Mitochondrial Dysfunction in Diabetic Kidney Disease via the PACS-2-Dependent Mechanism.Int J Biol Sci. 2024 Jan 1;20(2):569-584. doi: 10.7150/ijbs.89291. eCollection 2024. Int J Biol Sci. 2024. PMID: 38169625 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

