Type 2 diabetes as a protein misfolding disease
- PMID: 25998900
- PMCID: PMC4492843
- DOI: 10.1016/j.molmed.2015.04.005
Type 2 diabetes as a protein misfolding disease
Abstract
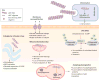
Type 2 diabetes (T2D) is a highly prevalent and chronic metabolic disorder. Recent evidence suggests that formation of toxic aggregates of the islet amyloid polypeptide (IAPP) might contribute to β-cell dysfunction and disease. However, the mechanism of protein aggregation and associated toxicity remains unclear. Misfolding, aggregation, and accumulation of diverse proteins in various organs is the hallmark of the group of protein misfolding disorders (PMDs), including highly prevalent illnesses affecting the central nervous system (CNS) such as Alzheimer's disease (AD) and Parkinson's disease (PD). In this review we discuss the current understanding of the mechanisms implicated in the formation of protein aggregates in the endocrine pancreas and associated toxicity in the light of the long-standing knowledge from neurodegenerative disorders associated with protein misfolding.
Keywords: amyloid; islet amyloid polypeptide; prions; protein misfolding; type 2 diabetes; β cells.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Prion-Like Protein Aggregates and Type 2 Diabetes.Cold Spring Harb Perspect Med. 2017 May 1;7(5):a024315. doi: 10.1101/cshperspect.a024315. Cold Spring Harb Perspect Med. 2017. PMID: 28159831 Free PMC article. Review.
-
Induction of IAPP amyloid deposition and associated diabetic abnormalities by a prion-like mechanism.J Exp Med. 2017 Sep 4;214(9):2591-2610. doi: 10.1084/jem.20161134. Epub 2017 Aug 1. J Exp Med. 2017. PMID: 28765400 Free PMC article.
-
Molecular interaction between type 2 diabetes and Alzheimer's disease through cross-seeding of protein misfolding.Mol Psychiatry. 2017 Sep;22(9):1327-1334. doi: 10.1038/mp.2016.230. Epub 2017 Jan 3. Mol Psychiatry. 2017. PMID: 28044060 Free PMC article.
-
α-Synuclein promotes IAPP fibril formation in vitro and β-cell amyloid formation in vivo in mice.Sci Rep. 2020 Nov 24;10(1):20438. doi: 10.1038/s41598-020-77409-z. Sci Rep. 2020. PMID: 33235246 Free PMC article.
-
The human islet amyloid polypeptide in protein misfolding disorders: Mechanisms of aggregation and interaction with biomembranes.Chem Phys Lipids. 2021 Jan;234:105010. doi: 10.1016/j.chemphyslip.2020.105010. Epub 2020 Nov 20. Chem Phys Lipids. 2021. PMID: 33227292 Review.
Cited by
-
Endoplasmic reticulum stress in diseases.MedComm (2020). 2024 Aug 26;5(9):e701. doi: 10.1002/mco2.701. eCollection 2024 Sep. MedComm (2020). 2024. PMID: 39188936 Free PMC article. Review.
-
The Future of Incretin-Based Approaches for Neurodegenerative Diseases in Older Adults: Which to Choose? A Review of their Potential Efficacy and Suitability.Drugs Aging. 2021 May;38(5):355-373. doi: 10.1007/s40266-021-00853-7. Epub 2021 Mar 19. Drugs Aging. 2021. PMID: 33738783 Review.
-
Diet affects glycosylation of serum proteins in women at risk for cardiometabolic disease.Eur J Nutr. 2021 Oct;60(7):3727-3741. doi: 10.1007/s00394-021-02539-7. Epub 2021 Mar 26. Eur J Nutr. 2021. PMID: 33770218 Free PMC article.
-
Changes in hippocampal volume, synaptic plasticity and amylin sensitivity in an animal model of type 2 diabetes are associated with increased vulnerability to amyloid-beta in advancing age.Front Aging Neurosci. 2024 Jun 21;16:1373477. doi: 10.3389/fnagi.2024.1373477. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38974903 Free PMC article.
-
Efficacy of IAPP suppression in mouse and human islets by GLP-1 analogue conjugated antisense oligonucleotide.Front Mol Biosci. 2023 Feb 6;10:1096286. doi: 10.3389/fmolb.2023.1096286. eCollection 2023. Front Mol Biosci. 2023. PMID: 36814640 Free PMC article.
References
-
- Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. - PubMed
-
- Hardy J, Gwinn-Hardy K. Genetic classification of primary neurodegenerative disease. Science. 1998;282:1075–1079. - PubMed
-
- Moreno-Gonzalez I, Soto C. Natural animal models of neurodegenerative protein misfolding diseases. Curr Pharm Des. 2012;18:1148–1158. - PubMed
-
- Butler AE, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

