Inhibition of hTERT/telomerase contributes to the antitumor activity of pristimerin in pancreatic ductal adenocarcinoma cells
- PMID: 25997419
- PMCID: PMC4484616
- DOI: 10.3892/or.2015.3989
Inhibition of hTERT/telomerase contributes to the antitumor activity of pristimerin in pancreatic ductal adenocarcinoma cells
Abstract
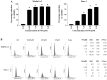
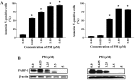
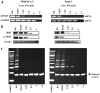
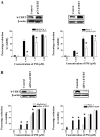
Pristimerin (PM) is a promising anticancer agent that has exhibited strong antiproliferative and apoptosis-inducing activity in various types of cancer cells. In the present study, we investigated the role of telomerase in mediating the antitumor activity of PM in pancreatic ductal adenocarcinoma (PDA) cells. PM inhibited cell proliferation, arrested cells in the G1 cell cycle phase and induced apoptosis in MiaPaCa-2 and Panc-1 PDA cells. These antitumor activities of PM correlated well with the inhibition of human telomerase reverse transcriptase (hTERT), the gene that codes for the catalytic subunit of telomerase complex. Gene knockin and knockdown approaches demonstrated that hTERT regulates the response of PDA cells to PM. PM inhibited hTERT expression by suppressing the transcription factors Sp1, c-Myc and NF-κB which control hTERT gene expression. PM also inhibited protein kinase Akt, which phosphorylates and facilitates hTERT nuclear importation and its telomerase activity. These findings identified hTERT (telomerase) as a potential therapeutic target of PM for the treatment of PDA.
Figures
Similar articles
-
Role of telomerase in anticancer activity of pristimerin in prostate cancer cells.J Exp Ther Oncol. 2015;11(1):41-9. J Exp Ther Oncol. 2015. PMID: 26259389
-
Pristimerin, a quinonemethide triterpenoid, induces apoptosis in pancreatic cancer cells through the inhibition of pro-survival Akt/NF-κB/mTOR signaling proteins and anti-apoptotic Bcl-2.Int J Oncol. 2014 May;44(5):1707-15. doi: 10.3892/ijo.2014.2325. Epub 2014 Mar 5. Int J Oncol. 2014. PMID: 24603988 Free PMC article.
-
Inhibition of cell proliferation and induction of apoptosis by oleanane triterpenoid (CDDO-Me) in pancreatic cancer cells is associated with the suppression of hTERT gene expression and its telomerase activity.Biochem Biophys Res Commun. 2012 Jun 15;422(4):561-7. doi: 10.1016/j.bbrc.2012.05.024. Epub 2012 May 16. Biochem Biophys Res Commun. 2012. PMID: 22609405 Free PMC article.
-
The inhibition of cell proliferation and induction of apoptosis in pancreatic ductal adenocarcinoma cells by verrucarin A, a macrocyclic trichothecene, is associated with the inhibition of Akt/NF-кB/mTOR prosurvival signaling.Int J Oncol. 2016 Sep;49(3):1139-47. doi: 10.3892/ijo.2016.3587. Epub 2016 Jun 29. Int J Oncol. 2016. PMID: 27573873
-
Telomerase reactivation is associated with hepatobiliary and pancreatic cancers.Hepatobiliary Pancreat Dis Int. 2020 Oct;19(5):420-428. doi: 10.1016/j.hbpd.2020.04.007. Epub 2020 May 1. Hepatobiliary Pancreat Dis Int. 2020. PMID: 32386990 Review.
Cited by
-
Cellular and Molecular Mechanisms of Pristimerin in Cancer Therapy: Recent Advances.Front Oncol. 2021 May 7;11:671548. doi: 10.3389/fonc.2021.671548. eCollection 2021. Front Oncol. 2021. PMID: 34026649 Free PMC article. Review.
-
Anticancer Potential and Molecular Targets of Pristimerin in Human Malignancies.Pharmaceuticals (Basel). 2024 Apr 30;17(5):578. doi: 10.3390/ph17050578. Pharmaceuticals (Basel). 2024. PMID: 38794148 Free PMC article. Review.
-
Terpenoids, Cannabimimetic Ligands, beyond the Cannabis Plant.Molecules. 2020 Mar 29;25(7):1567. doi: 10.3390/molecules25071567. Molecules. 2020. PMID: 32235333 Free PMC article. Review.
-
Pristimerin Exerts Pharmacological Effects Through Multiple Signaling Pathways: A Comprehensive Review.Drug Des Devel Ther. 2024 May 18;18:1673-1694. doi: 10.2147/DDDT.S460093. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38779590 Free PMC article. Review.
-
Telomerase Inhibitors from Natural Products and Their Anticancer Potential.Int J Mol Sci. 2017 Dec 21;19(1):13. doi: 10.3390/ijms19010013. Int J Mol Sci. 2017. PMID: 29267203 Free PMC article. Review.
References
-
- Pancreatic Cancer-National Cancer Institute, U.S. National Institutes of Health Cancer Gov. http:www.cancer.gov/cancer-topics/types/pancreatic. Retrieved 06-04-2010.
-
- Mulcahy MF, Wahl AO, Small W., Jr The current status of combined radiotherapy and chemotherapy for locally advanced or resected pancreas cancer. J Natl Compr Canc Netw. 2005;3:637–642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

