Detection of Plasma Protease Activity Using Microsphere-Cytometry Assays with E. coli Derived Substrates: VWF Proteolysis by ADAMTS13
- PMID: 25992814
- PMCID: PMC4436310
- DOI: 10.1371/journal.pone.0126556
Detection of Plasma Protease Activity Using Microsphere-Cytometry Assays with E. coli Derived Substrates: VWF Proteolysis by ADAMTS13
Abstract
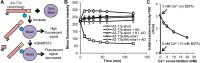
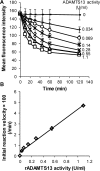
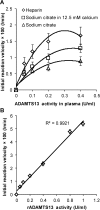
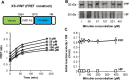
Protease levels in human blood are often prognostic indicators of inflammatory, thrombotic or oncogenic disorders. The measurement of such enzyme activities in substrate-based assays is complicated due to the low prevalence of these enzymes and steric hindrance of the substrates by the more abundant blood proteins. To address these limitations, we developed a molecular construct that is suitable for microsphere-cytometer based assays in the milieu of human blood plasma. In this proof of principle study, we demonstrate the utility of this substrate to measure metalloprotease ADAMTS13 activity. The substrate, expressed in E. coli as a fusion protein, contains the partial A2-domain of von Willebrand factor (VWF amino acids 1594-1670) that is mutated to include a single primary amine at the N-terminus and free cysteines at the C-terminus. N-terminus fluorescence conjugation was possible using NHS (N-hydroxysuccinimide) chemistry. Maleimide-PEG(Polyethylene glycol)n-biotin coupling at the C-terminus allowed biotinylation with variable PEG spacer lengths. Once bound to streptavidin-bearing microspheres, the substrate fluorescence signal decreased in proportion with ADAMTS13 concentration. Whereas recombinant ADAMTS13 activity could be quantified using substrates with all PEG repeat-lengths, only the construct with the longer 77 PEG-unit could quantify proteolysis in blood plasma. Using this longer substrate, plasma ADAMTS13 down to 5% of normal levels could be detected within 30 min. Such measurements could also be readily performed under conditions resembling hyperbilirubinemia. Enzyme catalytic activity was tuned by varying buffer calcium, with lower divalent ion concentrations enhancing cleavage. Overall, the study highlights the substrate design features important for the creation of efficient proteolysis assays in the setting of human plasma. In particular, it emphasizes the need to introduce PEG spacers in plasma-based experiments, a design attribute commonly ignored in immobilized peptide-substrate assays.
Conflict of interest statement
Figures

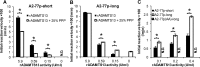
Similar articles
-
C2362F mutation gives rise to an ADAMTS13-resistant von Willebrand factor.Thromb Haemost. 2013 Jun;109(6):999-1006. doi: 10.1160/TH12-11-0808. Epub 2013 Feb 28. Thromb Haemost. 2013. PMID: 23446343
-
Amino acid residues Arg(659), Arg(660), and Tyr(661) in the spacer domain of ADAMTS13 are critical for cleavage of von Willebrand factor.Blood. 2010 Mar 18;115(11):2300-10. doi: 10.1182/blood-2009-07-235101. Epub 2010 Jan 14. Blood. 2010. PMID: 20075158 Free PMC article.
-
Probing ADAMTS13 substrate specificity using phage display.PLoS One. 2015 Apr 7;10(4):e0122931. doi: 10.1371/journal.pone.0122931. eCollection 2015. PLoS One. 2015. PMID: 25849793 Free PMC article.
-
[ADAMTS13, von Willebrand factor specific cleaving protease].Med Sci (Paris). 2011 Dec;27(12):1097-105. doi: 10.1051/medsci/20112712016. Epub 2011 Dec 23. Med Sci (Paris). 2011. PMID: 22192749 Review. French.
-
Recent advances in thrombotic thrombocytopenic purpura.Hematology Am Soc Hematol Educ Program. 2004:407-23. doi: 10.1182/asheducation-2004.1.407. Hematology Am Soc Hematol Educ Program. 2004. PMID: 15561695 Review.
Cited by
-
Degradation of the α-Carboxyl Terminus 11 Peptide: In Vivo and Ex Vivo Impacts of Time, Temperature, Inhibitors, and Gender in Rat.ACS Pharmacol Transl Sci. 2024 Apr 22;7(5):1624-1636. doi: 10.1021/acsptsci.4c00120. eCollection 2024 May 10. ACS Pharmacol Transl Sci. 2024. PMID: 38751644
-
Segmental outflow of aqueous humor in mouse and human.Exp Eye Res. 2017 May;158:59-66. doi: 10.1016/j.exer.2016.08.001. Epub 2016 Aug 3. Exp Eye Res. 2017. PMID: 27498226 Free PMC article. Review.
References
-
- Tirumalai RS, Chan KC, Prieto DA, Issaq HJ, Conrads TP, et al. Characterization of the low molecular weight human serum proteome. Mol Cell Proteomics. 2003; 2: 1096–1103. - PubMed
-
- Anderson NL, Polanski M, Pieper R, Gatlin T, Tirumalai RS, et al. The human plasma proteome: a nonredundant list developed by combination of four separate sources. Mol Cell Proteomics. 2004; 3: 311–326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

