Novel heparan sulfate-binding peptides for blocking herpesvirus entry
- PMID: 25992785
- PMCID: PMC4436313
- DOI: 10.1371/journal.pone.0126239
Novel heparan sulfate-binding peptides for blocking herpesvirus entry
Abstract
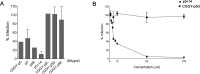
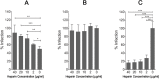
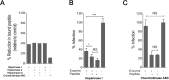
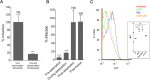
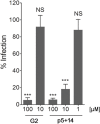
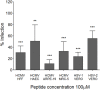
Human cytomegalovirus (HCMV) infection can lead to congenital hearing loss and mental retardation. Upon immune suppression, reactivation of latent HCMV or primary infection increases morbidity in cancer, transplantation, and late stage AIDS patients. Current treatments include nucleoside analogues, which have significant toxicities limiting their usefulness. In this study we screened a panel of synthetic heparin-binding peptides for their ability to prevent CMV infection in vitro. A peptide designated, p5+14 exhibited ~ 90% reduction in murine CMV (MCMV) infection. Because negatively charged, cell-surface heparan sulfate proteoglycans (HSPGs), serve as the attachment receptor during the adsorption phase of the CMV infection cycle, we hypothesized that p5+14 effectively competes for CMV adsorption to the cell surface resulting in the reduction in infection. Positively charged Lys residues were required for peptide binding to cell-surface HSPGs and reducing viral infection. We show that this inhibition was not due to a direct neutralizing effect on the virus itself and that the peptide blocked adsorption of the virus. The peptide also inhibited infection of other herpesviruses: HCMV and herpes simplex virus 1 and 2 in vitro, demonstrating it has broad-spectrum antiviral activity. Therefore, this peptide may offer an adjunct therapy for the treatment of herpes viral infections and other viruses that use HSPGs for entry.
Conflict of interest statement
Figures

Similar articles
-
Anticytomegalovirus Peptides Point to New Insights for CMV Entry Mechanisms and the Limitations of In Vitro Screenings.mSphere. 2019 Feb 13;4(1):e00586-18. doi: 10.1128/mSphere.00586-18. mSphere. 2019. PMID: 30760613 Free PMC article.
-
Peptide-derivatized dendrimers inhibit human cytomegalovirus infection by blocking virus binding to cell surface heparan sulfate.Antiviral Res. 2010 Mar;85(3):532-40. doi: 10.1016/j.antiviral.2010.01.003. Epub 2010 Jan 18. Antiviral Res. 2010. PMID: 20083141
-
Evasion of a Human Cytomegalovirus Entry Inhibitor with Potent Cysteine Reactivity Is Concomitant with the Utilization of a Heparan Sulfate Proteoglycan-Independent Route of Entry.J Virol. 2020 Mar 17;94(7):e02012-19. doi: 10.1128/JVI.02012-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31941787 Free PMC article.
-
Heparin-binding Peptides as Novel Therapies to Stop SARS-CoV-2 Cellular Entry and Infection.Mol Pharmacol. 2020 Nov;98(5):612-619. doi: 10.1124/molpharm.120.000098. Epub 2020 Sep 10. Mol Pharmacol. 2020. PMID: 32913137 Free PMC article. Review.
-
[Prevention and therapy of herpesvirus infections].Zentralbl Bakteriol Mikrobiol Hyg B. 1985 Feb;180(2-3):107-20. Zentralbl Bakteriol Mikrobiol Hyg B. 1985. PMID: 2986378 Review. German.
Cited by
-
A Preliminary Investigation on the Antiviral Activities of the Philippine Marshmint (Mentha arvensis) Leaf Extracts against Dengue Virus Serotype 2 In Vitro.Kobe J Med Sci. 2021 Dec 21;67(3):E98-E111. Kobe J Med Sci. 2021. PMID: 35367996 Free PMC article.
-
Pentosan Polysulfate Affords Pleotropic Protection to Multiple Cells and Tissues.Pharmaceuticals (Basel). 2023 Mar 13;16(3):437. doi: 10.3390/ph16030437. Pharmaceuticals (Basel). 2023. PMID: 36986536 Free PMC article. Review.
-
Tegument Protein pp150 Sequence-Specific Peptide Blocks Cytomegalovirus Infection.Viruses. 2021 Nov 15;13(11):2277. doi: 10.3390/v13112277. Viruses. 2021. PMID: 34835083 Free PMC article.
-
EXTL3 and NPC1 are mammalian host factors for Autographa californica multiple nucleopolyhedrovirus infection.Nat Commun. 2024 Sep 4;15(1):7711. doi: 10.1038/s41467-024-52193-w. Nat Commun. 2024. PMID: 39231976 Free PMC article.
-
Prevention of Herpesviridae Infections by Cationic PEGylated Carbosilane Dendrimers.Pharmaceutics. 2022 Feb 28;14(3):536. doi: 10.3390/pharmaceutics14030536. Pharmaceutics. 2022. PMID: 35335912 Free PMC article.
References
-
- Mocarski ES, Shenk T, Griffith P, Pass RF. Cytomegaloviruses In: Knipe DM, Howley PM, editors. Fields Virology (Sixth Edition), Sixth ed. Philadelphia: Lippincott Williams & Wilkins; 2013. p. 1960–2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

