Smad3 Signaling Promotes Fibrosis While Preserving Cardiac and Aortic Geometry in Obese Diabetic Mice
- PMID: 25985794
- PMCID: PMC4512850
- DOI: 10.1161/CIRCHEARTFAILURE.114.001963
Smad3 Signaling Promotes Fibrosis While Preserving Cardiac and Aortic Geometry in Obese Diabetic Mice
Abstract
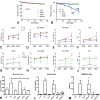
Background: Heart failure in diabetics is associated with cardiac hypertrophy, fibrosis and diastolic dysfunction. Activation of transforming growth factor-β/Smad3 signaling in the diabetic myocardium may mediate fibrosis and diastolic heart failure, while preserving matrix homeostasis. We hypothesized that Smad3 may play a key role in the pathogenesis of cardiovascular remodeling associated with diabetes mellitus and obesity.
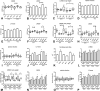
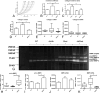
Methods and results: We generated leptin-resistant db/db Smad3 null mice and db/db Smad3+/- animals. Smad3 haploinsufficiency did not affect metabolic function in db/db mice, but protected from myocardial diastolic dysfunction, while causing left ventricular chamber dilation. Improved cardiac compliance and chamber dilation in db/db Smad3+/- animals were associated with decreased cardiomyocyte hypertrophy, reduced collagen deposition, and accentuated matrix metalloproteinase activity. Attenuation of hypertrophy and fibrosis in db/db Smad3+/- hearts was associated with reduced myocardial oxidative and nitrosative stress. db/db Smad3 null mice had reduced weight gain and decreased adiposity associated with attenuated insulin resistance, but also exhibited high early mortality, in part, because of spontaneous rupture of the ascending aorta. Ultrasound studies showed that both lean and obese Smad3 null animals had significant aortic dilation. Aortic dilation in db/db Smad3 null mice occurred despite reduced hypertension and was associated with perturbed matrix balance in the vascular wall.
Conclusions: Smad3 mediates diabetic cardiac hypertrophy, fibrosis, and diastolic dysfunction, while preserving normal cardiac geometry and maintaining the integrity of the vascular wall.
Keywords: TGF-β; diabetes mellitus; diabetic cardiomyopathies; fibrosis; obesity.
© 2015 American Heart Association, Inc.
Figures

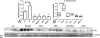

Similar articles
-
Fibroblast-specific TGF-β signaling mediates cardiac dysfunction, fibrosis, and hypertrophy in obese diabetic mice.Cardiovasc Res. 2024 Dec 14;120(16):2047-2063. doi: 10.1093/cvr/cvae210. Cardiovasc Res. 2024. PMID: 39373248
-
Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction.Am J Physiol Heart Circ Physiol. 2018 Oct 1;315(4):H934-H949. doi: 10.1152/ajpheart.00238.2018. Epub 2018 Jul 13. Am J Physiol Heart Circ Physiol. 2018. PMID: 30004258 Free PMC article.
-
Left atrial remodeling, hypertrophy, and fibrosis in mouse models of heart failure.Cardiovasc Pathol. 2017 Sep-Oct;30:27-37. doi: 10.1016/j.carpath.2017.06.003. Epub 2017 Jun 21. Cardiovasc Pathol. 2017. PMID: 28759817 Free PMC article.
-
Molecular mechanisms of cardiac pathology in diabetes - Experimental insights.Biochim Biophys Acta Mol Basis Dis. 2018 May;1864(5 Pt B):1949-1959. doi: 10.1016/j.bbadis.2017.10.035. Epub 2017 Nov 3. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29109032 Review.
-
Transforming growth factor beta (TGF-β) mediates cardiac fibrosis and induces diabetic cardiomyopathy.Diabetes Res Clin Pract. 2017 Nov;133:124-130. doi: 10.1016/j.diabres.2017.08.018. Epub 2017 Sep 1. Diabetes Res Clin Pract. 2017. PMID: 28934669 Review.
Cited by
-
AANG Prevents Smad3-dependent Diabetic Nephropathy by Restoring Pancreatic β-Cell Development in db/db Mice.Int J Biol Sci. 2022 Aug 29;18(14):5489-5502. doi: 10.7150/ijbs.72977. eCollection 2022. Int J Biol Sci. 2022. PMID: 36147472 Free PMC article.
-
Unraveling the Cardiac Matrix: From Diabetes to Heart Failure, Exploring Pathways and Potential Medications.Biomedicines. 2024 Jun 13;12(6):1314. doi: 10.3390/biomedicines12061314. Biomedicines. 2024. PMID: 38927520 Free PMC article. Review.
-
MicroRNAs regulating signaling pathways in cardiac fibrosis: potential role of the exercise training.Am J Physiol Heart Circ Physiol. 2024 Mar 1;326(3):H497-H510. doi: 10.1152/ajpheart.00410.2023. Epub 2023 Dec 8. Am J Physiol Heart Circ Physiol. 2024. PMID: 38063810 Review.
-
Unveiling the link between arsenic toxicity and diabetes: an in silico exploration into the role of transcription factors.Toxicol Res. 2024 Jul 18;40(4):653-672. doi: 10.1007/s43188-024-00255-y. eCollection 2024 Oct. Toxicol Res. 2024. PMID: 39345741
-
Combination Therapy of Alpha-Lipoic Acid, Gliclazide and Ramipril Protects Against Development of Diabetic Cardiomyopathy via Inhibition of TGF-β/Smad Pathway.Front Pharmacol. 2022 Mar 21;13:850542. doi: 10.3389/fphar.2022.850542. eCollection 2022. Front Pharmacol. 2022. PMID: 35401218 Free PMC article.
References
-
- Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. - PubMed
-
- Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;47:693–700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

