Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR
- PMID: 25981758
- PMCID: PMC4764353
- DOI: 10.1056/NEJMoa1409547
Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR
Abstract
Background: Cystic fibrosis is a life-limiting disease that is caused by defective or deficient cystic fibrosis transmembrane conductance regulator (CFTR) protein activity. Phe508del is the most common CFTR mutation.
Methods: We conducted two phase 3, randomized, double-blind, placebo-controlled studies that were designed to assess the effects of lumacaftor (VX-809), a CFTR corrector, in combination with ivacaftor (VX-770), a CFTR potentiator, in patients 12 years of age or older who had cystic fibrosis and were homozygous for the Phe508del CFTR mutation. In both studies, patients were randomly assigned to receive either lumacaftor (600 mg once daily or 400 mg every 12 hours) in combination with ivacaftor (250 mg every 12 hours) or matched placebo for 24 weeks. The primary end point was the absolute change from baseline in the percentage of predicted forced expiratory volume in 1 second (FEV1) at week 24.
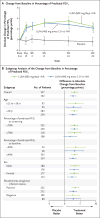
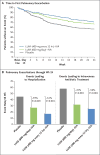
Results: A total of 1108 patients underwent randomization and received study drug. The mean baseline FEV1 was 61% of the predicted value. In both studies, there were significant improvements in the primary end point in both lumacaftor-ivacaftor dose groups; the difference between active treatment and placebo with respect to the mean absolute improvement in the percentage of predicted FEV1 ranged from 2.6 to 4.0 percentage points (P<0.001), which corresponded to a mean relative treatment difference of 4.3 to 6.7% (P<0.001). Pooled analyses showed that the rate of pulmonary exacerbations was 30 to 39% lower in the lumacaftor-ivacaftor groups than in the placebo group; the rate of events leading to hospitalization or the use of intravenous antibiotics was lower in the lumacaftor-ivacaftor groups as well. The incidence of adverse events was generally similar in the lumacaftor-ivacaftor and placebo groups. The rate of discontinuation due to an adverse event was 4.2% among patients who received lumacaftor-ivacaftor versus 1.6% among those who received placebo.
Conclusions: These data show that lumacaftor in combination with ivacaftor provided a benefit for patients with cystic fibrosis homozygous for the Phe508del CFTR mutation. (Funded by Vertex Pharmaceuticals and others; TRAFFIC and TRANSPORT ClinicalTrials.gov numbers, NCT01807923 and NCT01807949.).
Figures
Comment in
-
Another Beginning for Cystic Fibrosis Therapy.N Engl J Med. 2015 Jul 16;373(3):274-6. doi: 10.1056/NEJMe1504059. Epub 2015 May 17. N Engl J Med. 2015. PMID: 25981385 No abstract available.
-
A new chapter in therapy for cystic fibrosis.Lancet Respir Med. 2015 Jul;3(7):e20. doi: 10.1016/S2213-2600(15)00234-9. Epub 2015 Jun 24. Lancet Respir Med. 2015. PMID: 26117158 No abstract available.
-
Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR.N Engl J Med. 2015 Oct 29;373(18):1783-4. doi: 10.1056/NEJMc1510466. N Engl J Med. 2015. PMID: 26510034 No abstract available.
-
Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR.N Engl J Med. 2015 Oct 29;373(18):1783. doi: 10.1056/NEJMc1510466. N Engl J Med. 2015. PMID: 26510035 No abstract available.
-
Clinical Trials of Novel Treatments for Cystic Fibrosis.Am J Respir Crit Care Med. 2016 Mar 1;193(5):569-71. doi: 10.1164/rccm.201509-1734RR. Am J Respir Crit Care Med. 2016. PMID: 26765316 No abstract available.
-
[A milestone in cystic fibrosis therapy].MMW Fortschr Med. 2015 Nov 5;157(19):34. doi: 10.1007/s15006-015-3723-9. MMW Fortschr Med. 2015. PMID: 26953403 German. No abstract available.
Similar articles
-
A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase 2 randomised controlled trial.Lancet Respir Med. 2014 Jul;2(7):527-38. doi: 10.1016/S2213-2600(14)70132-8. Epub 2014 Jun 24. Lancet Respir Med. 2014. PMID: 24973281 Clinical Trial.
-
Efficacy and safety of lumacaftor and ivacaftor in patients aged 6-11 years with cystic fibrosis homozygous for F508del-CFTR: a randomised, placebo-controlled phase 3 trial.Lancet Respir Med. 2017 Jul;5(7):557-567. doi: 10.1016/S2213-2600(17)30215-1. Epub 2017 Jun 9. Lancet Respir Med. 2017. PMID: 28606620 Clinical Trial.
-
Efficacy and safety of lumacaftor/ivacaftor combination therapy in patients with cystic fibrosis homozygous for Phe508del CFTR by pulmonary function subgroup: a pooled analysis.Lancet Respir Med. 2016 Aug;4(8):617-626. doi: 10.1016/S2213-2600(16)30121-7. Epub 2016 Jun 10. Lancet Respir Med. 2016. PMID: 27298017 Free PMC article.
-
Lumacaftor/ivacaftor combination for cystic fibrosis patients homozygous for Phe508del-CFTR.Drugs Today (Barc). 2016 Apr;52(4):229-37. doi: 10.1358/dot.2016.52.4.2467205. Drugs Today (Barc). 2016. PMID: 27252987 Free PMC article. Review.
-
Correctors (specific therapies for class II CFTR mutations) for cystic fibrosis.Cochrane Database Syst Rev. 2018 Aug 2;8(8):CD010966. doi: 10.1002/14651858.CD010966.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 Dec 17;12:CD010966. doi: 10.1002/14651858.CD010966.pub3 PMID: 30070364 Free PMC article. Updated. Review.
Cited by
-
Modulator Therapy in Cystic Fibrosis Patients with cis Variants in F508del Complex Allele: A Short-Term Observational Case Series.J Pers Med. 2022 Aug 31;12(9):1421. doi: 10.3390/jpm12091421. J Pers Med. 2022. PMID: 36143206 Free PMC article.
-
Novel Anti-Inflammatory Approaches for Cystic Fibrosis Lung Disease: Identification of Molecular Targets and Design of Innovative Therapies.Front Pharmacol. 2020 Jul 23;11:1096. doi: 10.3389/fphar.2020.01096. eCollection 2020. Front Pharmacol. 2020. PMID: 32848733 Free PMC article. Review.
-
Personalized medicine approaches in cystic fibrosis related pancreatitis.Am J Transl Res. 2022 Oct 15;14(10):7612-7620. eCollection 2022. Am J Transl Res. 2022. PMID: 36398272 Free PMC article.
-
From Ivacaftor to Triple Combination: A Systematic Review of Efficacy and Safety of CFTR Modulators in People with Cystic Fibrosis.Int J Mol Sci. 2020 Aug 16;21(16):5882. doi: 10.3390/ijms21165882. Int J Mol Sci. 2020. PMID: 32824306 Free PMC article.
-
RNA Sequencing: A Potentiator of Discovery-based Research.Am J Respir Cell Mol Biol. 2019 Nov;61(5):558-559. doi: 10.1165/rcmb.2019-0156ED. Am J Respir Cell Mol Biol. 2019. PMID: 31091961 Free PMC article. No abstract available.
References
-
- US CF Foundation, Johns Hopkins University, the Hospital for Sick Children. The clinical and functional translation of CFTR (CFTR2) 2011 ( http://cftr2.org)
-
- Cystic Fibrosis Foundation. Patient registry: annual data report, 2012. Bethesda, MD: Cystic Fibrosis Foundation; 2013. ( http://www.cff.org/UploadedFiles/research/ClinicalResearch/PatientRegist...)
-
- European Cystic Fibrosis Patient Registry: annual data report, 2010. Denmark: European Cystic Fibrosis Society; 2014. ( https://www.ecfs.eu/files/webfm/webfiles/File/ecfs_registry/ECFSPR_Repor...)
-
- The molecular genetic epidemiology of cystic fibrosis: report of a joint meeting of WHO/ECFTN/ICF(M)A/ECFS. Genoa, Italy: World Health Organization; Jun, 2002. p. 24.
-
- O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373:1891–904. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1TR000165/TR/NCATS NIH HHS/United States
- UL1TR000423/TR/NCATS NIH HHS/United States
- P30 DK072482/DK/NIDDK NIH HHS/United States
- UL1RR025741/RR/NCRR NIH HHS/United States
- UL1 TR000427/TR/NCATS NIH HHS/United States
- UL1TR000062/TR/NCATS NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- UL1 TR000165/TR/NCATS NIH HHS/United States
- P30 DK089507/DK/NIDDK NIH HHS/United States
- P30 DK027651/DK/NIDDK NIH HHS/United States
- UL1 RR025741/RR/NCRR NIH HHS/United States
- DK072482/DK/NIDDK NIH HHS/United States
- UL1 TR000062/TR/NCATS NIH HHS/United States
- P30DK089507/DK/NIDDK NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1TR000439/TR/NCATS NIH HHS/United States
- UL1TR001079/TR/NCATS NIH HHS/United States
- UL1 TR000423/TR/NCATS NIH HHS/United States
- DK027651/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
