Fibroblast Growth Factor 9 Regulation by MicroRNAs Controls Lung Development and Links DICER1 Loss to the Pathogenesis of Pleuropulmonary Blastoma
- PMID: 25978641
- PMCID: PMC4433140
- DOI: 10.1371/journal.pgen.1005242
Fibroblast Growth Factor 9 Regulation by MicroRNAs Controls Lung Development and Links DICER1 Loss to the Pathogenesis of Pleuropulmonary Blastoma
Abstract
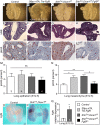
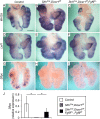
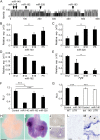
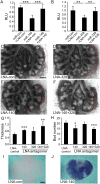
Pleuropulmonary Blastoma (PPB) is the primary neoplastic manifestation of a pediatric cancer predisposition syndrome that is associated with several diseases including cystic nephroma, Wilms tumor, neuroblastoma, rhabdomyosarcoma, medulloblastoma, and ovarian Sertoli-Leydig cell tumor. The primary pathology of PPB, epithelial cysts with stromal hyperplasia and risk for progression to a complex primitive sarcoma, is associated with familial heterozygosity and lesion-associated epithelial loss-of-heterozygosity of DICER1. It has been hypothesized that loss of heterozygosity of DICER1 in lung epithelium is a non-cell autonomous etiology of PPB and a critical pathway that regulates lung development; however, there are no known direct targets of epithelial microRNAs (miRNAs) in the lung. Fibroblast Growth Factor 9 (FGF9) is expressed in the mesothelium and epithelium during lung development and primarily functions to regulate lung mesenchyme; however, there are no known mechanisms that regulate FGF9 expression during lung development. Using mouse genetics and molecular phenotyping of human PPB tissue, we show that FGF9 is overexpressed in lung epithelium in the initial multicystic stage of Type I PPB and that in mice lacking epithelial Dicer1, or induced to overexpress epithelial Fgf9, increased Fgf9 expression results in pulmonary mesenchymal hyperplasia and a multicystic architecture that is histologically and molecularly indistinguishable from Type I PPB. We further show that miR-140 is expressed in lung epithelium, regulates epithelial Fgf9 expression, and regulates pseudoglandular stages of lung development. These studies identify an essential miRNA-FGF9 pathway for lung development and a non-cell autonomous signaling mechanism that contributes to the mesenchymal hyperplasia that is characteristic of Type I PPB.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Cell- and developmental stage-specific Dicer1 ablation in the lung epithelium models cystic pleuropulmonary blastoma.J Pathol. 2015 May;236(1):41-52. doi: 10.1002/path.4500. Epub 2015 Jan 20. J Pathol. 2015. PMID: 25500911 Free PMC article.
-
Deletion of Yy1 in mouse lung epithelium unveils molecular mechanisms governing pleuropulmonary blastoma pathogenesis.Dis Model Mech. 2020 Nov 6;13(12):dmm045989. doi: 10.1242/dmm.045989. Dis Model Mech. 2020. PMID: 33158935 Free PMC article.
-
DICER1 pleuropulmonary blastoma familial tumour predisposition syndrome: What the paediatric urologist needs to know.J Pediatr Urol. 2016 Feb;12(1):5-10. doi: 10.1016/j.jpurol.2015.08.012. Epub 2015 Sep 26. J Pediatr Urol. 2016. PMID: 26454454 Review.
-
Ovarian sex cord-stromal tumors, pleuropulmonary blastoma and DICER1 mutations: a report from the International Pleuropulmonary Blastoma Registry.Gynecol Oncol. 2011 Aug;122(2):246-50. doi: 10.1016/j.ygyno.2011.03.024. Epub 2011 Apr 17. Gynecol Oncol. 2011. PMID: 21501861 Free PMC article.
-
Wilms tumor, pleuropulmonary blastoma, and DICER1: case report and literature review.World J Surg Oncol. 2018 Aug 10;16(1):164. doi: 10.1186/s12957-018-1469-4. World J Surg Oncol. 2018. PMID: 30097050 Free PMC article. Review.
Cited by
-
FGF9 and FGF10 activate distinct signaling pathways to direct lung epithelial specification and branching.Sci Signal. 2020 Mar 3;13(621):eaay4353. doi: 10.1126/scisignal.aay4353. Sci Signal. 2020. PMID: 32127497 Free PMC article.
-
Umbilical cord blood exosomes from very preterm infants with bronchopulmonary dysplasia aggravate lung injury in mice.Sci Rep. 2023 May 27;13(1):8648. doi: 10.1038/s41598-023-35620-8. Sci Rep. 2023. PMID: 37244977 Free PMC article.
-
MicroRNA in late lung development and bronchopulmonary dysplasia: the need to demonstrate causality.Mol Cell Pediatr. 2016 Dec;3(1):19. doi: 10.1186/s40348-016-0047-5. Epub 2016 May 23. Mol Cell Pediatr. 2016. PMID: 27216745 Free PMC article. Review.
-
Pleuropulmonary Blastoma: Evolution of an Entity as an Entry into a Familial Tumor Predisposition Syndrome.Pediatr Dev Pathol. 2015 Nov-Dec;18(6):504-11. doi: 10.2350/15-10-1732-OA.1. Epub 2015 Dec 23. Pediatr Dev Pathol. 2015. PMID: 26698637 Free PMC article.
-
miR-187-3p increases gemcitabine sensitivity in breast cancer cells by targeting FGF9 expression.Exp Ther Med. 2020 Aug;20(2):952-960. doi: 10.3892/etm.2020.8770. Epub 2020 May 19. Exp Ther Med. 2020. PMID: 32765654 Free PMC article.
References
-
- Colvin JS, White A, Pratt SJ, Ornitz DM. Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development. 2001;128:2095–106. - PubMed
-
- White AC, Xu J, Yin Y, Smith C, Schmid G, Ornitz DM. FGF9 and SHH signaling coordinate lung growth and development through regulation of distinct mesenchymal domains. Development. 2006;133(8):1507–17. Epub 2006/03/17. - PubMed
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

