Melatonin reverses H2 O2 -induced premature senescence in mesenchymal stem cells via the SIRT1-dependent pathway
- PMID: 25975679
- PMCID: PMC4523475
- DOI: 10.1111/jpi.12250
Melatonin reverses H2 O2 -induced premature senescence in mesenchymal stem cells via the SIRT1-dependent pathway
Abstract
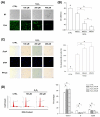
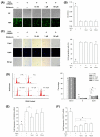
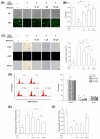
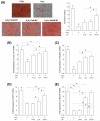
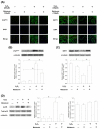
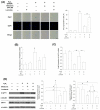
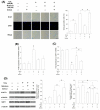
Mesenchymal stem cells (MSCs) represent an attractive source for stem cell-based regenerative therapy, but they are vulnerable to oxidative stress-induced premature senescence in pathological conditions. We previously reported antioxidant and antiarthritic effects of melatonin on MSCs against proinflammatory cytokines. In this study, we hypothesized that melatonin could protect MSCs from premature senescence induced by hydrogen peroxide (H2 O2 ) via the silent information regulator type 1 (SIRT1)-dependent pathway. In response to H2 O2 at a sublethal concentration of 200 μm, human bone marrow-derived MSCs (BM-MSCs) underwent growth arrest and cellular senescence. Treatment with melatonin before H2 O2 exposure cannot significantly prevent premature senescence; however, treatment with melatonin subsequent to H2 O2 exposure successfully reversed the senescent phenotypes of BM-MSCs in a dose-dependent manner. This result was made evident by improved cell proliferation, decreased senescence-associated β-galactosidase activity, and the improved entry of proliferating cells into the S phase. In addition, treatment with 100 μm melatonin restored the osteogenic differentiation potential of BM-MSCs that was inhibited by H2 O2 -induced premature senescence. We also found that melatonin attenuated the H2 O2 -stimulated phosphorylation of p38 mitogen-activated protein kinase, decreased expression of the senescence-associated protein p16(INK) (4α) , and increased SIRT1. Further molecular experiments revealed that luzindole, a nonselective antagonist of melatonin receptors, blocked melatonin-mediated antisenescence effects. Inhibition of SIRT1 by sirtinol counteracted the protective effects of melatonin, suggesting that melatonin reversed the senescence in cells through the SIRT1-dependent pathway. Together, these findings lay new ground for understanding oxidative stress-induced premature senescence and open perspectives for therapeutic applications of melatonin in stem cell-based regenerative medicine.
Keywords: SIRT1; hydrogen peroxide; melatonin; mesenchymal stem cells; senescence.
© 2015 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures

Similar articles
-
SIRT1-dependent anti-senescence effects of cell-deposited matrix on human umbilical cord mesenchymal stem cells.J Tissue Eng Regen Med. 2018 Feb;12(2):e1008-e1021. doi: 10.1002/term.2422. Epub 2017 Jun 20. J Tissue Eng Regen Med. 2018. PMID: 28107614 Free PMC article.
-
Spontaneous up-regulation of SIRT1 during osteogenesis contributes to stem cells' resistance to oxidative stress.J Cell Biochem. 2018 Jun;119(6):4928-4944. doi: 10.1002/jcb.26730. Epub 2018 Mar 7. J Cell Biochem. 2018. PMID: 29380418 Free PMC article.
-
Alcohol Induces Cellular Senescence and Impairs Osteogenic Potential in Bone Marrow-Derived Mesenchymal Stem Cells.Alcohol Alcohol. 2017 May 1;52(3):289-297. doi: 10.1093/alcalc/agx006. Alcohol Alcohol. 2017. PMID: 28339869 Free PMC article.
-
Senescence suppressors: their practical importance in replicative lifespan extension in stem cells.Cell Mol Life Sci. 2014 Nov;71(21):4207-19. doi: 10.1007/s00018-014-1685-1. Epub 2014 Jul 23. Cell Mol Life Sci. 2014. PMID: 25052377 Free PMC article. Review.
-
Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence.Biochem Pharmacol. 2012 Nov 15;84(10):1332-9. doi: 10.1016/j.bcp.2012.06.031. Epub 2012 Jul 14. Biochem Pharmacol. 2012. PMID: 22796566 Free PMC article. Review.
Cited by
-
Age and sex modify cellular proliferation responses to oxidative stress and glucocorticoid challenges in baboon cells.Geroscience. 2021 Aug;43(4):2067-2085. doi: 10.1007/s11357-021-00395-1. Epub 2021 Jun 5. Geroscience. 2021. PMID: 34089175 Free PMC article.
-
The Role of Antioxidants in the Interplay between Oxidative Stress and Senescence.Antioxidants (Basel). 2022 Jun 22;11(7):1224. doi: 10.3390/antiox11071224. Antioxidants (Basel). 2022. PMID: 35883714 Free PMC article. Review.
-
Mesenchymal Stem Cell Senescence during Aging:From Mechanisms to Rejuvenation Strategies.Aging Dis. 2023 Oct 1;14(5):1651-1676. doi: 10.14336/AD.2023.0208. Aging Dis. 2023. PMID: 37196126 Free PMC article. Review.
-
Radiation-Induced Dual Oxidase Upregulation in Rat Heart Tissues: Protective Effect of Melatonin.Medicina (Kaunas). 2019 Jun 27;55(7):317. doi: 10.3390/medicina55070317. Medicina (Kaunas). 2019. PMID: 31252673 Free PMC article.
-
Women's health and night shift work: Potential targets for future strategies in breast cancer (Review).Biomed Rep. 2021 Dec;15(6):98. doi: 10.3892/br.2021.1474. Epub 2021 Sep 24. Biomed Rep. 2021. PMID: 34667595 Free PMC article. Review.
References
-
- PITTENGER MF, MACKAY AM, BECK SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- OREFFO RO, COOPER C, MASON C, et al. Mesenchymal stem cells: lineage, plasticity, and skeletal therapeutic potential. Stem Cell Rev. 2005;1:169–178. - PubMed
-
- VIDAL MA, WALKER NJ, NAPOLI E, et al. Evaluation of senescence in mesenchymal stem cells isolated from equine bone marrow, adipose tissue, and umbilical cord tissue. Stem Cells Dev. 2012;21:273–283. - PubMed
-
- BRANDL A, MEYER M, BECHMANN V, et al. Oxidative stress induces senescence in human mesenchymal stem cells. Exp Cell Res. 2011;317:1541–1547. - PubMed
-
- DUMONT P, BURTON M, CHEN QM, et al. Induction of replicative senescence biomarkers by sublethal oxidative stresses in normal human fibroblast. Free Radic Biol Med. 2000;28:361–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

