Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: population based cohort study
- PMID: 25975601
- PMCID: PMC4431352
- DOI: 10.1136/bmj.h2102
Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: population based cohort study
Abstract
Objective: To provide absolute and relative risk estimates of neonatal abstinence syndrome (NAS) based on duration and timing of prescription opioid use during pregnancy in the presence or absence of additional NAS risk factors of history of opioid misuse or dependence, misuse of other substances, non-opioid psychotropic drug use, and smoking.
Design: Observational cohort study.
Setting: Medicaid data from 46 US states.
Participants: Pregnant women filling at least one prescription for an opioid analgesic at any time during pregnancy for whom opioid exposure characteristics including duration of therapy: short term (<30 days) or long term (≥ 30 days); timing of use: early use (only in the first two trimesters) or late use (extending into the third trimester); and cumulative dose (in morphine equivalent milligrams) were assessed.
Main outcome measure: Diagnosis of NAS in liveborn infants.
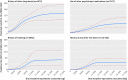
Results: 1705 cases of NAS were identified among 290,605 pregnant women filling opioid prescriptions, corresponding to an absolute risk of 5.9 per 1000 deliveries (95% confidence interval 5.6 to 6.2). Long term opioid use during pregnancy resulted in higher absolute risk of NAS per 1000 deliveries in the presence of additional risk factors of known opioid misuse (220.2 (200.8 to 241.0)), alcohol or other drug misuse (30.8 (26.1 to 36.0)), exposure to other psychotropic medications (13.1 (10.6 to 16.1)), and smoking (6.6 (4.3 to 9.6)) than in the absence of any of these risk factors (4.2 (3.3 to 5.4)). The corresponding risk estimates for short term use were 192.0 (175.8 to 209.3), 7.0 (6.0 to 8.2), 2.0 (1.5 to 2.6), 1.5 (1.0 to 2.0), and 0.7 (0.6 to 0.8) per 1000 deliveries, respectively. In propensity score matched analyses, long term prescription opioid use compared with short term use and late use compared with early use in pregnancy demonstrated greater risk of NAS (risk ratios 2.05 (95% confidence interval 1.81 to 2.33) and 1.24 (1.12 to 1.38), respectively).
Conclusions: Use of prescription opioids during pregnancy is associated with a low absolute risk of NAS in the absence of additional risk factors. Long term use compared with short term use and late use compared with early use of prescription opioids are associated with increased NAS risk independent of additional risk factors.
© Desai et al 2015.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Similar articles
-
Risk of neonatal drug withdrawal after intrauterine co-exposure to opioids and psychotropic medications: cohort study.BMJ. 2017 Aug 2;358:j3326. doi: 10.1136/bmj.j3326. BMJ. 2017. PMID: 28768628 Free PMC article.
-
Prevalence of Maternal-Risk Factors Related to Neonatal Abstinence Syndrome in a Commercial Claims Database: 2011-2015.Pharmacotherapy. 2019 Oct;39(10):1005-1011. doi: 10.1002/phar.2315. Epub 2019 Aug 22. Pharmacotherapy. 2019. PMID: 31361913
-
Predicting treatment for neonatal abstinence syndrome in infants born to women maintained on opioid agonist medication.Addiction. 2012 Nov;107 Suppl 1(0 1):45-52. doi: 10.1111/j.1360-0443.2012.04038.x. Addiction. 2012. PMID: 23106926 Free PMC article. Clinical Trial.
-
Opioids in pregnancy and neonatal abstinence syndrome.Semin Perinatol. 2015 Nov;39(7):561-5. doi: 10.1053/j.semperi.2015.08.013. Semin Perinatol. 2015. PMID: 26452318 Free PMC article. Review.
-
Methadone dose and neonatal abstinence syndrome-systematic review and meta-analysis.Addiction. 2010 Dec;105(12):2071-84. doi: 10.1111/j.1360-0443.2010.03120.x. Epub 2010 Sep 15. Addiction. 2010. PMID: 20840198 Review.
Cited by
-
Late Pregnancy β Blocker Exposure and Risks of Neonatal Hypoglycemia and Bradycardia.Pediatrics. 2016 Sep;138(3):e20160731. doi: 10.1542/peds.2016-0731. Pediatrics. 2016. PMID: 27577580 Free PMC article.
-
Prenatal Exposure to Opioids and Neurodevelopmental Disorders in Children: A Bayesian Mediation Analysis.Am J Epidemiol. 2024 Feb 5;193(2):308-322. doi: 10.1093/aje/kwad183. Am J Epidemiol. 2024. PMID: 37671942 Free PMC article.
-
Long-term Healthcare Utilization by Medicaid Enrolled Children with Neonatal Abstinence Syndrome.J Pediatr. 2020 Jun;221:55-63.e6. doi: 10.1016/j.jpeds.2020.02.077. J Pediatr. 2020. PMID: 32446493 Free PMC article.
-
Neonatal Abstinence Syndrome: Trend and Expenditure in Louisiana Medicaid, 2003-2013.Matern Child Health J. 2017 Jul;21(7):1479-1487. doi: 10.1007/s10995-017-2268-1. Matern Child Health J. 2017. PMID: 28168591
-
Antipsychotic Medication Use Among Publicly Insured Pregnant Women in the United States.Psychiatr Serv. 2017 Nov 1;68(11):1112-1119. doi: 10.1176/appi.ps.201600408. Epub 2017 Jun 15. Psychiatr Serv. 2017. PMID: 28617210 Free PMC article.
References
-
- Jansson LM, Velez M. Neonatal abstinence syndrome. Curr Opin Pediatr 2012;24:252-8. - PubMed
-
- Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA 2012;307:1934-40. - PubMed
-
- Creanga AA, Sabel JC, Ko JY, et al. Maternal drug use and its effect on neonates: a population-based study in Washington State. Obstet Gynecol 2012;119:924-33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical