Roux-en-Y Gastric Bypass Acutely Decreases Protein Carbonylation and Increases Expression of Mitochondrial Biogenesis Genes in Subcutaneous Adipose Tissue
- PMID: 25975200
- PMCID: PMC4648364
- DOI: 10.1007/s11695-015-1708-5
Roux-en-Y Gastric Bypass Acutely Decreases Protein Carbonylation and Increases Expression of Mitochondrial Biogenesis Genes in Subcutaneous Adipose Tissue
Abstract
Background: Mitochondrial dysfunction in adipose tissue has been implicated as a pathogenic step in the development of type 2 diabetes mellitus (T2DM). In adipose tissue, chronic nutrient overload results in mitochondria driven increased reactive oxygen species (ROS) leading to carbonylation of proteins that impair mitochondrial function and downregulation of key genes linked to mitochondrial biogenesis. In patients with T2DM, Roux-en-Y gastric bypass (RYGB) surgery leads to improvements in glycemic profile prior to significant weight loss. Consequently, we hypothesized that improved glycemia early after RYGB would be paralleled by decreased protein carbonylation and increased expression of genes related to mitochondrial biogenesis in adipose tissue.
Methods: To evaluate this hypothesis, 16 obese individuals were studied before and 7-8 days following RYGB and adjustable gastric banding (AGB). Subcutaneous adipose tissue was obtained pre- and post-bariatric surgery as well as from eight healthy, non-obese individual controls.
Results: Prior to surgery, adipose tissue expression of PGC1α, NRF1, Cyt C, and eNOS (but not Tfam) showed significantly lower expression in the obese bariatric surgery group when compared to lean controls (p < 0.05). Following RYGB, but not after AGB, patients showed significant decrease in HOMA-IR, reduction in adipose protein carbonylation, and increased expression of genes linked to mitochondrial biogenesis.
Conclusions: These results suggest that rapid reduction in protein carbonylation and increased mitochondrial biogenesis may explain postoperative metabolic improvements following RYGB.
Keywords: Adipose tissue; Adjustable gastric banding; Mitochondrial biogenesis; Protein carbonylation; Roux-en-Y gastric bypass.
Figures
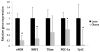
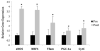
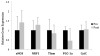



Similar articles
-
Differential Gene Expression of Subcutaneous Adipose Tissue among Lean, Obese, and after RYGB (Different Timepoints): Systematic Review and Analysis.Nutrients. 2022 Nov 21;14(22):4925. doi: 10.3390/nu14224925. Nutrients. 2022. PMID: 36432612 Free PMC article. Review.
-
Long-Term Gestational Hypoxia Modulates Expression of Key Genes Governing Mitochondrial Function in the Perirenal Adipose of the Late Gestation Sheep Fetus.Reprod Sci. 2015 Jun;22(6):654-63. doi: 10.1177/1933719114561554. Epub 2014 Dec 10. Reprod Sci. 2015. PMID: 25504105 Free PMC article.
-
Altered promoter methylation of PDK4, IL1 B, IL6, and TNF after Roux-en Y gastric bypass.Surg Obes Relat Dis. 2014 Jul-Aug;10(4):671-8. doi: 10.1016/j.soard.2013.12.019. Epub 2014 Jan 28. Surg Obes Relat Dis. 2014. PMID: 24837562
-
Effect of Roux-en-Y gastric bypass on liver mitochondrial dynamics in a rat model of obesity.Physiol Rep. 2018 Feb;6(4):e13600. doi: 10.14814/phy2.13600. Physiol Rep. 2018. PMID: 29464885 Free PMC article.
-
Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass.Dan Med J. 2015 Apr;62(4):B5057. Dan Med J. 2015. PMID: 25872541 Review.
Cited by
-
Oxygenation of adipose tissue: A human perspective.Acta Physiol (Oxf). 2020 Jan;228(1):e13298. doi: 10.1111/apha.13298. Epub 2019 Jun 2. Acta Physiol (Oxf). 2020. PMID: 31077538 Free PMC article. Review.
-
Adipose tissue gene expression is differentially regulated with different rates of weight loss in overweight and obese humans.Int J Obes (Lond). 2017 Feb;41(2):309-316. doi: 10.1038/ijo.2016.201. Epub 2016 Nov 14. Int J Obes (Lond). 2017. PMID: 27840413 Clinical Trial.
-
Differential Gene Expression of Subcutaneous Adipose Tissue among Lean, Obese, and after RYGB (Different Timepoints): Systematic Review and Analysis.Nutrients. 2022 Nov 21;14(22):4925. doi: 10.3390/nu14224925. Nutrients. 2022. PMID: 36432612 Free PMC article. Review.
-
Altered mitochondrial function in insulin-deficient and insulin-resistant states.J Clin Invest. 2018 Aug 31;128(9):3671-3681. doi: 10.1172/JCI120843. Epub 2018 Aug 31. J Clin Invest. 2018. PMID: 30168804 Free PMC article. Review.
-
RYGB Is More Effective than VSG at Protecting Mice from Prolonged High-Fat Diet Exposure: An Occasion to Roll Up Our Sleeves?Obes Surg. 2021 Jul;31(7):3227-3241. doi: 10.1007/s11695-021-05389-8. Epub 2021 Apr 15. Obes Surg. 2021. PMID: 33856636
References
-
- Malandrucco I, Pasqualetti P, Giordani I, et al. Very-low-calorie diet: a quick therapeutic tool to improve b cell function in morbidly obese patients with type 2 diabetes. Am J Clin Nutr. 2012;95:609–13. - PubMed
-
- Boudina S, Graham TE. Mitochondrial function/dysfunction in white adipose tissue. Exp Physiol. 2014;99(9):1168–78. doi:10.1113/expphysiol.2014.081414. Epub 2014 Aug 15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

