Single-Cell Characterization of in vitro Migration and Interaction Dynamics of T Cells Expanded with IL-2 and IL-7
- PMID: 25972868
- PMCID: PMC4412128
- DOI: 10.3389/fimmu.2015.00196
Single-Cell Characterization of in vitro Migration and Interaction Dynamics of T Cells Expanded with IL-2 and IL-7
Abstract
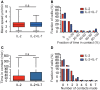
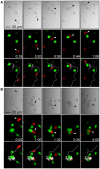
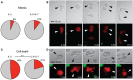
T cells are pivotal in the immune defense against cancers and infectious agents. To mount an effector response against cancer cells, T cells need to migrate to the cancer-site, engage in contacts with cancer cells, and perform their effector functions. Adoptive T cell therapy is an effective strategy as treatment of complications such as relapse or opportunistic infections after hematopoietic stem cell transplantations. This requires a sufficient amount of cells that are able to expand and respond to tumor or viral antigens. The cytokines interleukin (IL)-2 and IL-7 drive T cell differentiation, proliferation, and survival and are commonly used to expand T cells ex vivo. Here, we have used microchip-based live-cell imaging to follow the migration of individual T cells, their interactions with allogeneic monocytes, cell division, and apoptosis for extended periods of time; something that cannot be achieved by commonly used methods. Our data indicate that cells grown in IL-7 + IL-2 had similar migration and contact dynamics as cells grown in IL-2 alone. However, the addition of IL-7 decreased cell death creating a more viable cell population, which should be beneficial when preparing cells for immunotherapy.
Keywords: IL-2; IL-7; T cell; fluorescence; live-cell imaging; microchip; microscopy; single-cell analysis.
Figures


Similar articles
-
Ex vivo expansion of dendritic-cell-activated antigen-specific CD4+ T cells with anti-CD3/CD28, interleukin-7, and interleukin-15: potential for adoptive T cell immunotherapy.Clin Immunol. 2006 Apr;119(1):21-31. doi: 10.1016/j.clim.2005.11.003. Epub 2006 Jan 9. Clin Immunol. 2006. PMID: 16406844
-
Ex vivo cytokine activation of peripheral blood stem cells: a potential role for adoptive cellular immunotherapy.J Hematother Stem Cell Res. 2001 Apr;10(2):283-90. doi: 10.1089/15258160151135006. J Hematother Stem Cell Res. 2001. PMID: 11359675
-
Ex vivo characterization of γδ T-cell repertoire in patients after adoptive transfer of Vγ9Vδ2 T cells expressing the interleukin-2 receptor β-chain and the common γ-chain.Cytotherapy. 2013 Apr;15(4):481-91. doi: 10.1016/j.jcyt.2012.12.004. Epub 2013 Feb 5. Cytotherapy. 2013. PMID: 23391461 Clinical Trial.
-
Therapeutic gene modified cell based cancer vaccines.Gene. 2013 Aug 10;525(2):200-7. doi: 10.1016/j.gene.2013.03.056. Epub 2013 Apr 6. Gene. 2013. PMID: 23566846 Review.
-
Ex vivo expansion of hematopoietic cells and their clinical use.Haematologica. 1998 Sep;83(9):824-48. Haematologica. 1998. PMID: 9825579 Review.
Cited by
-
Bridging the gap: microfluidic devices for short and long distance cell-cell communication.Lab Chip. 2017 Mar 14;17(6):1009-1023. doi: 10.1039/c6lc01367h. Lab Chip. 2017. PMID: 28205652 Free PMC article. Review.
-
Inflammation-on-a-Chip: Probing the Immune System Ex Vivo.Trends Biotechnol. 2018 Sep;36(9):923-937. doi: 10.1016/j.tibtech.2018.03.011. Epub 2018 May 1. Trends Biotechnol. 2018. PMID: 29728272 Free PMC article. Review.
-
Interleukin-2 induces extracellular matrix synthesis and TGF-β2 expression in retinal pigment epithelial cells.Dev Growth Differ. 2019 Sep;61(7-8):410-418. doi: 10.1111/dgd.12630. Epub 2019 Oct 13. Dev Growth Differ. 2019. PMID: 31608440 Free PMC article.
-
Sctensor detects many-to-many cell-cell interactions from single cell RNA-sequencing data.BMC Bioinformatics. 2023 Nov 7;24(1):420. doi: 10.1186/s12859-023-05490-y. BMC Bioinformatics. 2023. PMID: 37936079 Free PMC article.
-
Human Vγ9Vδ2 T cell expansion and their cytotoxic responses against cholangiocarcinoma.Sci Rep. 2024 Jan 14;14(1):1291. doi: 10.1038/s41598-024-51794-1. Sci Rep. 2024. PMID: 38221530 Free PMC article.
References
-
- Cole DJ, Taubenberger JK, Pockaj BA, Yannelli JR, Carter C, Carrasquillo J, et al. Histopathological analysis of metastatic melanoma deposits in patients receiving adoptive immunotherapy with tumor-infiltrating lymphocytes. Cancer Immunol Immunother (1994) 38(5):299–303.10.1007/BF01525507 - DOI - PMC - PubMed
-
- Rosenberg SA, Aebersold P, Cornetta K, Kasid A, Morgan RA, Moen R, et al. Gene transfer into humans – immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med (1990) 323(9):570–8.10.1056/NEJM199008303230904 - DOI - PubMed
-
- Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein–Barr virus-induced lymphoma in allogeneic transplant recipients. Blood (1998) 92(5):1549–55. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

