Therapeutic inhibition of TRF1 impairs the growth of p53-deficient K-RasG12V-induced lung cancer by induction of telomeric DNA damage
- PMID: 25971796
- PMCID: PMC4520658
- DOI: 10.15252/emmm.201404497
Therapeutic inhibition of TRF1 impairs the growth of p53-deficient K-RasG12V-induced lung cancer by induction of telomeric DNA damage
Abstract
Telomeres are considered anti-cancer targets, as telomere maintenance above a minimum length is necessary for cancer growth. Telomerase abrogation in cancer-prone mouse models, however, only decreased tumor growth after several mouse generations when telomeres reach a critically short length, and this effect was lost upon p53 mutation. Here, we address whether induction of telomere uncapping by inhibition of the TRF1 shelterin protein can effectively block cancer growth independently of telomere length. We show that genetic Trf1 ablation impairs the growth of p53-null K-Ras(G12V)-induced lung carcinomas and increases mouse survival independently of telomere length. This is accompanied by induction of telomeric DNA damage, apoptosis, decreased proliferation, and G2 arrest. Long-term whole-body Trf1 deletion in adult mice did not impact on mouse survival and viability, although some mice showed a moderately decreased cellularity in bone marrow and blood. Importantly, inhibition of TRF1 binding to telomeres by small molecules blocks the growth of already established lung carcinomas without affecting mouse survival or tissue function. Thus, induction of acute telomere uncapping emerges as a potential new therapeutic target for lung cancer.
Keywords: TRF1; cancer; drug development; shelterin; telomeres.
© 2015 The Authors. Published under the terms of the CC BY 4.0 license.
Figures
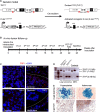
Genetic model. Trf1lox and K-RasLSLGV12 alleles are depicted before and after Cre-mediated excision.
In vivo imaging schedule. Eight- to ten-week-old mice were intratracheally infected with adeno-Cre, mice were analyzed every 2 weeks by computerized tomography (CT), and 22 weeks post-infection, a positron emission tomography (PET) was performed. Mice were sacrificed 24 weeks post-infection for further histological analysis.
TRF1 immunofluorescence of the lungs. Notice the absence and presence of TRF1 signal in the carcinomas and surrounding healthy tissue of Trf1Δ/Δ mice, respectively.
Analysis of Trf1 excision by PCR. Notice the completed excision in carcinomas of Trf1lox/lox lungs.
Detection of β-galactosidase activity in the lungs as a surrogate marker of oncogenic K-RasG12V expression.
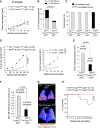
Tumor growth curve of Trf1+/+ K-Ras+/G12V p53+/+ and Trf1Δ/Δ K-Ras+/G12V p53+/+ measured by computed tomography (CT).
Quantification of the number and size of Trf1+/+ K-Ras+/G12V p53+/+ and Trf1Δ/Δ K-Ras+/G12V p53+/+ carcinomas at death point.
Percentage of tumors that have deleted Trf1 quantified by TRF1 immunofluorescence after mice had been sacrificed. Post-mortem analysis of Trf1 deletion in each tumor revealed that none of the Trf1lox/lox K-Ras+/G12V p53+/+ ones had excised Trf1.
Tumor growth curve and tumor growth slope of Trf1+/+ K-Ras+/G12V p53−/− and Trf1Δ/Δ K-Ras+/G12V p53−/− measured by CT.
Tumor maximal section of Trf1+/+ K-Ras+/G12V p53−/− and Trf1Δ/Δ K-Ras+/G12V p53−/− lungs measured by histological analysis before death point by CT.
Maximum 18F-FDG-glucose uptake by Trf1+/+ K-Ras+/G12V p53−/− and Trf1Δ/Δ K-Ras+/G12V p53−/− tumors 22 weeks after infection by positron emission tomography (PET).
Representative PET-CT image of Trf1+/+ K-Ras+/G12V p53−/− and Trf1Δ/Δ K-Ras+/G12V p53−/− lungs.
Survival curve of Trf1+/+ K-Ras+/G12V p53−/− and Trf1Δ/Δ K-Ras+/G12V p53−/− mice.
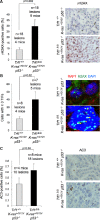
Percentage of cells showing γH2AX foci in carcinomas of the indicated genotypes (left panel). Representative images of γH2AX immunohistochemistry (right panel).
Percentage of cells showing 3 or more γH2AX and RAP1 colocalizing foci (TIFs) (left panel). Representative images of γH2AX and RAP1 double immunofluorescence (right panel). Yellow arrowheads: colocalization of γH2AX and RAP1.
Percentage of active caspase-3 (AC3)-positive cells (left panel). Representative images of AC3 immunohistochemistry (right panel).
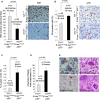
Percentage of Ki67-positive cells in the carcinomas of the indicated genotypes (left panel). Representative images of Ki67 immunohistochemistry (right panel).
Percentage of pH3-positive cells in the carcinomas of the indicated genotypes (left panel). Representative images of pH3 immunohistochemistry (right panel). Red arrowheads: pH3-positive cells.
Percentage of giant nuclei in the carcinomas of the indicated genotype.
Percentage of anaphase bridges out of total anaphases in the carcinomas of the indicated genotypes.
Representative images of giant nuclei, multilobulated nuclei, anaphase bridges, and multipolar mitoses. Red arrowheads indicate the corresponding mitotic aberrations indicated in the image.
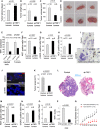
A–C The latency (A), volume (B), and weight (C) of subcutaneous tumors generated by control and Trf1-downregulated K-RasG12V-transformed lung cells in athymic mice.
D Representative images of the subcutaneous tumors.
E Trf1 expression levels measured by qPCR in the injected cell line and in the generated subcutaneous tumors.
F–H Number of Ki67-positive (F), number of γH2AX-positive (G), and number of active caspase-3-positive (H) cells per field in the subcutaneous tumors.
I Representative images of aberrant giant nuclei and anaphase bridges in the Trf1-downregulated subcutaneous tumors compared to the normal nuclei of control tumors.
J TRF1 immunofluorescence shows the downregulation of Trf1 in lung tumors of the mice intravenously injected with control and Trf1-downregulated cells.
K Tumor area measured in the lungs of the mice intravenously injected with control and Trf1-downregulated cells.
L Representative images of the lungs colonized by control and Trf1-downregulated cells, respectively.
M–O Number of Ki67-positive (M), number of γH2AX-positive (N), and number of active caspase-3-positive (O) cells per field in the lung tumors.
P Trf1 expression levels measured by qPCR in the A549 cell line infected either with sh-scrambled or sh-Trf1.
Q Growth of A549-derived tumors.
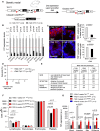
Trf1lox/lox hUBC-CreERT2 genetic mouse model.
Trf1 expression levels in the indicated tissues of wild-type and Trf1lox/lox hUBC-CreERT2 mice subjected to a tamoxifen-containing diet for 7 weeks.
Representative images of TRF1 immunofluorescence and quantification of the percentage of TRF1-positive cells in skin and intestine sections of wild-type and Trf1lox/lox hUBC-CreERT2 mice subjected to a tamoxifen-containing diet for 7 weeks.
Survival curve of wild-type and Trf1lox/lox hUBC-CreERT2 mice subjected to a tamoxifen-containing diet for 7 weeks.
Quantification of the histological alterations observed in tamoxifen-treated Trf1lox/lox hUBC-CreERT2 mice and 4 months after tamoxifen retrieval compared to their wild-type counterparts.
Quantification of blood cell populations in wild-type and Trf1lox/lox hUBC-CreERT2 mice subjected to a tamoxifen-containing diet for 7 weeks and after 3 weeks and 4 months of tamoxifen retrieval.
Trf1 expression levels in blood, intestine, skin, and bone marrow of Trf1lox/lox hUBC-CreERT2 mice subjected to a tamoxifen-containing diet either for 7 weeks or for 6 months and after 3 weeks tamoxifen retrieval compared to wild-type mice.
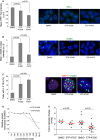
Quantification of TRF1 levels by immunofluorescence in lung tumor-derived cell line treated with DMSO, with 10 μM ETP-47228 (24 h), and with 10 μM ETP-47037 (48 h). Representative images are shown to the right.
Quantification of γH2AX levels by immunofluorescence in lung tumor-derived cell line treated with DMSO, with ETP-47228 (24 h), and with ETP-47037 (48 h). Representative images are shown to the right.
Quantification of telomere-induced foci (TIFs) by double immunofluorescence with anti-RAP1 and anti-γH2AX antibodies. Representative images are shown to the right. White arrowheads: colocalization of γH2AX and RAP1.
Effect of different ETP-47228 and ETP-47037 concentrations during 24 h on proliferation in lung tumor-derived cell line relative to the growth of DMSO-treated cells.
Tumor growth quantification in allograft model ETP-47037 or with ETP-47228.
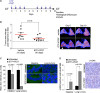
Schematic representation of the ETP-47037 treatment protocol. Mice with already developed lung carcinomas were subjected to computerized tomography (CT) measurements before the start of the treatment. ETP-47037 was given at a dose of 75 mg/kg body weight by oral gavage 8 days out of the ten that the experiment lasted as indicated. At the end of the treatment, a CT was performed for quantification of tumor area and mice were sacrificed for further histological and molecular analysis. Control mice were similarly treated with vehicle.
Quantification of tumor growth relative to initial tumor size. Representative CT images are shown to the right. The white arrowhead indicates a tumor within a highly inflammatory region.
Quantification of TRF1 levels by immunofluorescence in intestine and lung tumors samples of mice treated with vehicle or with ETP-47037 for 10 days. Representative images are shown to the right (n = 4).
Number of cells showing γH2AX foci in intestine and lung tumors samples of mice treated with vehicle or with ETP-47037 for 10 days. Representative images are shown to the right (n = 4).
A–C Quantification of the number of (A) Ki67-, (B) pan-nuclear p-H3 pattern-, and (C) foci p-H3 pattern-positive cells in untreated and ETP-470037-treated lung carcinomas. The data represent the mean values obtained for three mice in each group. Error bars represent standard errors.
D Representative Ki67 and p-H3 images.
E Representative H&E images of intestine samples corresponding to untreated and ETP-47037-treated animals. High-magnification images are shown to the right indicating the presence of normal mitosis, giant multinucleated and aberrant mitotic figures.
F Representative H&E images of bone marrow and skin samples corresponding to untreated and ETP-47037-treated animals. High-magnification images are shown indicating the presence of necrosis, hemosiderosis, multinucleated cells, and giant nuclei. Bone marrow showed moderated aplasia.
G Telomere length in untreated and ETP-47037-treated lung tumor samples. Representative images are shown to the right.
Similar articles
-
Multiple cancer pathways regulate telomere protection.EMBO Mol Med. 2019 Jul;11(7):e10292. doi: 10.15252/emmm.201910292. Epub 2019 Jun 13. EMBO Mol Med. 2019. PMID: 31273934 Free PMC article.
-
Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice.Genes Dev. 2009 Sep 1;23(17):2060-75. doi: 10.1101/gad.543509. Epub 2009 Aug 13. Genes Dev. 2009. PMID: 19679647 Free PMC article.
-
Inhibition of TRF1 Telomere Protein Impairs Tumor Initiation and Progression in Glioblastoma Mouse Models and Patient-Derived Xenografts.Cancer Cell. 2017 Nov 13;32(5):590-607.e4. doi: 10.1016/j.ccell.2017.10.006. Cancer Cell. 2017. PMID: 29136505
-
Role of Pin2/TRF1 in telomere maintenance and cell cycle control.J Cell Biochem. 2003 May 1;89(1):19-37. doi: 10.1002/jcb.10496. J Cell Biochem. 2003. PMID: 12682905 Review.
-
Inhibiting TRF1 upstream signaling pathways to target telomeres in cancer cells.EMBO Mol Med. 2019 Jul;11(7):e10845. doi: 10.15252/emmm.201910845. Epub 2019 Jun 13. EMBO Mol Med. 2019. PMID: 31273935 Free PMC article. Review.
Cited by
-
Targeting telomerase for cancer therapy.Oncogene. 2020 Sep;39(36):5811-5824. doi: 10.1038/s41388-020-01405-w. Epub 2020 Jul 30. Oncogene. 2020. PMID: 32733068 Free PMC article. Review.
-
Genetically Engineered Mouse Models of K-Ras-Driven Lung and Pancreatic Tumors: Validation of Therapeutic Targets.Cold Spring Harb Perspect Med. 2018 May 1;8(5):a031542. doi: 10.1101/cshperspect.a031542. Cold Spring Harb Perspect Med. 2018. PMID: 28778964 Free PMC article. Review.
-
Structural Features of Nucleoprotein CST/Shelterin Complex Involved in the Telomere Maintenance and Its Association with Disease Mutations.Cells. 2020 Feb 4;9(2):359. doi: 10.3390/cells9020359. Cells. 2020. PMID: 32033110 Free PMC article. Review.
-
Therapeutic Targets in Telomerase and Telomere Biology of Cancers.Indian J Clin Biochem. 2020 Apr;35(2):135-146. doi: 10.1007/s12291-020-00876-8. Epub 2020 Mar 10. Indian J Clin Biochem. 2020. PMID: 32226245 Free PMC article. Review.
-
Fundamental mechanisms of telomerase action in yeasts and mammals: understanding telomeres and telomerase in cancer cells.Open Biol. 2017 Mar;7(3):160338. doi: 10.1098/rsob.160338. Open Biol. 2017. PMID: 28330934 Free PMC article. Review.
References
-
- Ambrogio C, Carmona FJ, Vidal A, Falcone M, Nieto P, Romero OA, Puertas S, Vizoso M, Nadal E, Poggio T, et al. Modeling lung cancer evolution and preclinical response by orthotopic mouse allografts. Cancer Res. 2014;74:5978–5988. - PubMed
-
- Bellon M, Datta A, Brown M, Pouliquen JF, Couppie P, Kazanji M, Nicot C. Increased expression of telomere length regulating factors TRF1, TRF2 and TIN2 in patients with adult T-cell leukemia. Int J Cancer. 2006;119:2090–2097. - PubMed
-
- Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol. 2007;3:640–649. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

