CIP2A is a candidate therapeutic target in clinically challenging prostate cancer cell populations
- PMID: 25965834
- PMCID: PMC4637312
- DOI: 10.18632/oncotarget.3875
CIP2A is a candidate therapeutic target in clinically challenging prostate cancer cell populations
Abstract
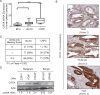
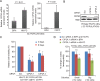
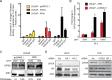
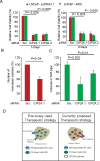
Residual androgen receptor (AR)-signaling and presence of cancer stem-like cells (SCs) are the two emerging paradigms for clinically challenging castration-resistant prostate cancer (CRPC). Therefore, identification of AR-target proteins that are also overexpressed in the cancer SC population would be an attractive therapeutic approach.Our analysis of over three hundred clinical samples and patient-derived prostate epithelial cultures (PPECs), revealed Cancerous inhibitor of protein phosphatase 2A (CIP2A) as one such target. CIP2A is significantly overexpressed in both hormone-naïve prostate cancer (HN-PC) and CRPC patients . CIP2A is also overexpressed, by 3- and 30-fold, in HN-PC and CRPC SCs respectively. In vivo binding of the AR to the intronic region of CIP2A and its functionality in the AR-moderate and AR-high expressing LNCaP cell-model systems is also demonstrated. Further, we show that AR positively regulates CIP2A expression, both at the mRNA and protein level. Finally, CIP2A depletion reduced cell viability and colony forming efficiency of AR-independent PPECs as well as AR-responsive LNCaP cells, in which anchorage-independent growth is also impaired.These findings identify CIP2A as a common denominator for AR-signaling and cancer SC functionality, highlighting its potential therapeutic significance in the most clinically challenging prostate pathology: castration-resistant prostate cancer.
Keywords: CIP2A; androgen receptor; cancer stem-like cells; castration-resistant prostate cancer.
Conflict of interest statement
None.
Figures
Similar articles
-
Galectin-3 Is Implicated in Tumor Progression and Resistance to Anti-androgen Drug Through Regulation of Androgen Receptor Signaling in Prostate Cancer.Anticancer Res. 2017 Jan;37(1):125-134. doi: 10.21873/anticanres.11297. Anticancer Res. 2017. PMID: 28011482
-
Therapeutic Potential of Leelamine, a Novel Inhibitor of Androgen Receptor and Castration-Resistant Prostate Cancer.Mol Cancer Ther. 2018 Oct;17(10):2079-2090. doi: 10.1158/1535-7163.MCT-18-0117. Epub 2018 Jul 20. Mol Cancer Ther. 2018. PMID: 30030299 Free PMC article.
-
Src promotes castration-recurrent prostate cancer through androgen receptor-dependent canonical and non-canonical transcriptional signatures.Oncotarget. 2017 Feb 7;8(6):10324-10347. doi: 10.18632/oncotarget.14401. Oncotarget. 2017. PMID: 28055971 Free PMC article.
-
Androgen receptor: what we know and what we expect in castration-resistant prostate cancer.Int Urol Nephrol. 2018 Oct;50(10):1753-1764. doi: 10.1007/s11255-018-1964-0. Epub 2018 Aug 20. Int Urol Nephrol. 2018. PMID: 30128923 Review.
-
Androgen receptor signaling in castration-resistant prostate cancer: a lesson in persistence.Endocr Relat Cancer. 2016 Dec;23(12):T179-T197. doi: 10.1530/ERC-16-0422. Epub 2016 Oct 31. Endocr Relat Cancer. 2016. PMID: 27799360 Review.
Cited by
-
RAS and PP2A activities converge on epigenetic gene regulation.Life Sci Alliance. 2023 Mar 1;6(5):e202301928. doi: 10.26508/lsa.202301928. Print 2023 May. Life Sci Alliance. 2023. PMID: 36858798 Free PMC article.
-
Pre-Clinical Study Evaluating Novel Protein Phosphatase 2A Activators as Therapeutics for Neuroblastoma.Cancers (Basel). 2022 Apr 13;14(8):1952. doi: 10.3390/cancers14081952. Cancers (Basel). 2022. PMID: 35454859 Free PMC article.
-
PP2A inhibition as a novel therapeutic target in castration-resistant prostate cancer.Tumour Biol. 2015 Aug;36(8):5753-5. doi: 10.1007/s13277-015-3849-5. Epub 2015 Aug 4. Tumour Biol. 2015. PMID: 26234767
-
Reciprocal regulation of CIP2A and AR expression in prostate cancer cells.Discov Oncol. 2022 Sep 13;13(1):87. doi: 10.1007/s12672-022-00552-8. Discov Oncol. 2022. PMID: 36098827 Free PMC article.
-
Synergistic Interaction of HOXB13 and CIP2A Predisposes to Aggressive Prostate Cancer.Clin Cancer Res. 2018 Dec 15;24(24):6265-6276. doi: 10.1158/1078-0432.CCR-18-0444. Epub 2018 Sep 4. Clin Cancer Res. 2018. PMID: 30181389 Free PMC article.
References
-
- Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nature genetics. 1995;9:401–406. - PubMed
-
- Waltering KK, Urbanucci A, Visakorpi T. Androgen receptor (AR) aberrations in castration-resistant prostate cancer. Molecular and cellular endocrinology. 2012;360:38–43. - PubMed
-
- Hara T, Miyazaki J, Araki H, Yamaoka M, Kanzaki N, Kusaka M, Miyamoto M. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer research. 2003;63:149–153. - PubMed
-
- Visakorpi T. Novel endocrine aspects of prostate cancer. Molecular and cellular endocrinology. 2012;360:1–2. - PubMed
-
- Rane JK, Pellacani D, Maitland NJ. Advanced prostate cancer--a case for adjuvant differentiation therapy. Nature reviews Urology. 2012;9:595–602. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

