Stromal androgen receptor regulates the composition of the microenvironment to influence prostate cancer outcome
- PMID: 25965833
- PMCID: PMC4599261
- DOI: 10.18632/oncotarget.3873
Stromal androgen receptor regulates the composition of the microenvironment to influence prostate cancer outcome
Erratum in
-
Erratum: Stromal androgen receptor regulates the composition of the microenvironment to influence prostate cancer outcome.Oncotarget. 2015 Nov 3;6(34):36923. doi: 10.18632/oncotarget.6263. Oncotarget. 2015. PMID: 26577649 Free PMC article. No abstract available.
Abstract
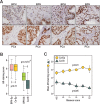
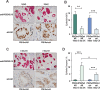
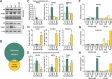
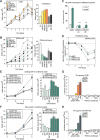
Androgen receptor (AR) signaling in stromal cells is important in prostate cancer, yet the mechanisms underpinning stromal AR contribution to disease development and progression remain unclear. Using patient-matched benign and malignant prostate samples, we show a significant association between low AR levels in cancer associated stroma and increased prostate cancer-related death at one, three and five years post-diganosis, and in tissue recombination models with primary prostate cancer cells that low stromal AR decreases castration-induced apoptosis. AR-regulation was found to be different in primary human fibroblasts isolated from adjacent to cancerous and non-cancerous prostate epithelia, and to represent altered activation of myofibroblast pathways involved in cell cycle, adhesion, migration, and the extracellular matrix (ECM). Without AR signaling, the fibroblast-derived ECM loses the capacity to promote attachment of both myofibroblasts and cancer cells, is less able to prevent cell-matrix disruption, and is less likely to impede cancer cell invasion. AR signaling in prostate cancer stroma appears therefore to alter patient outcome by maintaining an ECM microenvironment inhibitory to cancer cell invasion. This paper provides comprehensive insight into AR signaling in the non-epithelial prostate microenvironment, and a resource from which the prognostic and therapeutic implications of stromal AR levels can be further explored.
Keywords: androgen receptor; extracellular matrix; fibroblasts; prostate cancer; stroma.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures

Similar articles
-
Androgen receptor as a regulator of ZEB2 expression and its implications in epithelial-to-mesenchymal transition in prostate cancer.Endocr Relat Cancer. 2014 May 8;21(3):473-86. doi: 10.1530/ERC-13-0514. Print 2014 Jun. Endocr Relat Cancer. 2014. PMID: 24812058
-
Hic-5 influences genomic and non-genomic actions of the androgen receptor in prostate myofibroblasts.Mol Cell Endocrinol. 2014 Mar 25;384(1-2):185-99. doi: 10.1016/j.mce.2014.01.004. Epub 2014 Jan 17. Mol Cell Endocrinol. 2014. PMID: 24440747
-
Stromal TGF-β signaling induces AR activation in prostate cancer.Oncotarget. 2014 Nov 15;5(21):10854-69. doi: 10.18632/oncotarget.2536. Oncotarget. 2014. PMID: 25333263 Free PMC article.
-
Progesterone receptor in the prostate: A potential suppressor for benign prostatic hyperplasia and prostate cancer.J Steroid Biochem Mol Biol. 2017 Feb;166:91-96. doi: 10.1016/j.jsbmb.2016.04.008. Epub 2016 Apr 25. J Steroid Biochem Mol Biol. 2017. PMID: 27125450 Review.
-
Stromal androgen receptor roles in the development of normal prostate, benign prostate hyperplasia, and prostate cancer.Am J Pathol. 2015 Feb;185(2):293-301. doi: 10.1016/j.ajpath.2014.10.012. Epub 2014 Nov 26. Am J Pathol. 2015. PMID: 25432062 Free PMC article. Review.
Cited by
-
Evidence of the Link between Stroma Remodeling and Prostate Cancer Prognosis.Cancers (Basel). 2024 Sep 21;16(18):3215. doi: 10.3390/cancers16183215. Cancers (Basel). 2024. PMID: 39335188 Free PMC article. Review.
-
Sex differences in cancer and immunotherapy outcomes: the role of androgen receptor.Front Immunol. 2024 May 28;15:1416941. doi: 10.3389/fimmu.2024.1416941. eCollection 2024. Front Immunol. 2024. PMID: 38863718 Free PMC article. Review.
-
AR loss in prostate cancer stroma mediated by NF-κB and p38-MAPK signaling disrupts stromal morphogen production.Oncogene. 2024 Jun;43(27):2092-2103. doi: 10.1038/s41388-024-03064-7. Epub 2024 May 20. Oncogene. 2024. PMID: 38769192
-
Distinct mesenchymal cell states mediate prostate cancer progression.Nat Commun. 2024 Jan 8;15(1):363. doi: 10.1038/s41467-023-44210-1. Nat Commun. 2024. PMID: 38191471 Free PMC article.
-
Cellular specificity of androgen receptor, coregulators, and pioneer factors in prostate cancer.Endocr Oncol. 2022 Sep 8;2(1):R112-R131. doi: 10.1530/EO-22-0065. eCollection 2022 Jan. Endocr Oncol. 2022. PMID: 37435460 Free PMC article. Review.
References
-
- American Cancer Society I. Cancer Facts & Figures 2012. American Cancer Society; 2012.
-
- Amling CL, Blute ML, Bergstralh EJ, Seay TM, Slezak J, Zincke H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol. 2000;164:101–105. - PubMed
-
- Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, Partin AW. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. - PubMed
-
- Saadi A, Shannon NB, Lao-Sirieix P, O'Donovan M, Walker E, Clemons NJ, Hardwick JS, Zhang C, Das M, Save V, Novelli M, Balkwill F, Fitzgerald RC. Stromal genes discriminate preinvasive from invasive disease, predict outcome, and highlight inflammatory pathways in digestive cancers. Proc Natl Acad Sci U S A. 2010;107:2177–2182. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

