The Voltage-Gated Proton Channel: A Riddle, Wrapped in a Mystery, inside an Enigma
- PMID: 25964989
- PMCID: PMC4736506
- DOI: 10.1021/acs.biochem.5b00353
The Voltage-Gated Proton Channel: A Riddle, Wrapped in a Mystery, inside an Enigma
Abstract
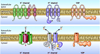
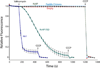
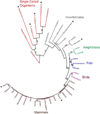
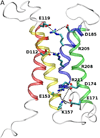
The main properties of the voltage-gated proton channel (HV1) are described in this review, along with what is known about how the channel protein structure accomplishes its functions. Just as protons are unique among ions, proton channels are unique among ion channels. Their four transmembrane helices sense voltage and the pH gradient and conduct protons exclusively. Selectivity is achieved by the unique ability of H3O(+) to protonate an Asp-Arg salt bridge. Pathognomonic sensitivity of gating to the pH gradient ensures HV1 channel opening only when acid extrusion will result, which is crucial to most of its biological functions. An exception occurs in dinoflagellates in which influx of H(+) through HV1 triggers the bioluminescent flash. Pharmacological interventions that promise to ameliorate cancer, asthma, brain damage in ischemic stroke, Alzheimer's disease, autoimmune diseases, and numerous other conditions await future progress.
Figures






Similar articles
-
Analysis of an electrostatic mechanism for ΔpH dependent gating of the voltage-gated proton channel, HV1, supports a contribution of protons to gating charge.Biochim Biophys Acta Bioenerg. 2021 Nov 1;1862(11):148480. doi: 10.1016/j.bbabio.2021.148480. Epub 2021 Aug 5. Biochim Biophys Acta Bioenerg. 2021. PMID: 34363792 Free PMC article. Review.
-
Voltage-gated proton channels.Cell Mol Life Sci. 2008 Aug;65(16):2554-73. doi: 10.1007/s00018-008-8056-8. Cell Mol Life Sci. 2008. PMID: 18463791 Free PMC article. Review.
-
Hydrophobic gasket mutation produces gating pore currents in closed human voltage-gated proton channels.Proc Natl Acad Sci U S A. 2019 Sep 17;116(38):18951-18961. doi: 10.1073/pnas.1905462116. Epub 2019 Aug 28. Proc Natl Acad Sci U S A. 2019. PMID: 31462498 Free PMC article.
-
Voltage-dependent structural models of the human Hv1 proton channel from long-timescale molecular dynamics simulations.Proc Natl Acad Sci U S A. 2020 Jun 16;117(24):13490-13498. doi: 10.1073/pnas.1920943117. Epub 2020 May 27. Proc Natl Acad Sci U S A. 2020. PMID: 32461356 Free PMC article.
-
Dimer interaction in the Hv1 proton channel.Proc Natl Acad Sci U S A. 2020 Aug 25;117(34):20898-20907. doi: 10.1073/pnas.2010032117. Epub 2020 Aug 11. Proc Natl Acad Sci U S A. 2020. PMID: 32788354 Free PMC article.
Cited by
-
H+ channels in embryonic Biomphalaria glabrata cell membranes: Putative roles in snail host-schistosome interactions.PLoS Negl Trop Dis. 2017 Mar 20;11(3):e0005467. doi: 10.1371/journal.pntd.0005467. eCollection 2017 Mar. PLoS Negl Trop Dis. 2017. PMID: 28319196 Free PMC article.
-
Role of the Voltage-Gated Proton Channel Hv1 in Nervous Systems.Neurosci Bull. 2023 Jul;39(7):1157-1172. doi: 10.1007/s12264-023-01053-6. Epub 2023 Apr 8. Neurosci Bull. 2023. PMID: 37029856 Free PMC article. Review.
-
Quantitative insights into the mechanism of proton conduction and selectivity for the human voltage-gated proton channel Hv1.Proc Natl Acad Sci U S A. 2024 Sep 17;121(38):e2407479121. doi: 10.1073/pnas.2407479121. Epub 2024 Sep 11. Proc Natl Acad Sci U S A. 2024. PMID: 39259593
-
The Role of Proton Transport in Gating Current in a Voltage Gated Ion Channel, as Shown by Quantum Calculations.Sensors (Basel). 2018 Sep 18;18(9):3143. doi: 10.3390/s18093143. Sensors (Basel). 2018. PMID: 30231473 Free PMC article.
-
The voltage-gated proton channel Hv1 plays a detrimental role in contusion spinal cord injury via extracellular acidosis-mediated neuroinflammation.Brain Behav Immun. 2021 Jan;91:267-283. doi: 10.1016/j.bbi.2020.10.005. Epub 2020 Oct 8. Brain Behav Immun. 2021. PMID: 33039662 Free PMC article.
References
-
- de Grotthuss CJT. Mémoire sur la décomposition de l'eau et des corps qu'elle tient en dissolution à l'aide de l'électricité galvanique. Annales de Chimie LVIII. 1806:54–74.
-
- de Grotthuss CJT. Memoir on the decomposition of water and of the bodies that it holds in solution by means of galvanic electricity. 1805. Biochim. Biophys. Acta. 2006;1757:871–875. - PubMed
-
- Markovitch O, Chen H, Izvekov S, Paesani F, Voth GA, Agmon N. Special pair dance and partner selection: elementary steps in proton transport in liquid water. J. Phys. Chem. B. 2008;112:9456–9466. - PubMed
-
- Danneel H. Notizüber Ionengeschwindigkeiten. Zeitschrift für Elektrochemie und angewandte physikalische Chemie. 1905;11:249–252.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

