How do K-RAS-activated cells evade cellular defense mechanisms?
- PMID: 25961920
- PMCID: PMC4761642
- DOI: 10.1038/onc.2015.153
How do K-RAS-activated cells evade cellular defense mechanisms?
Abstract
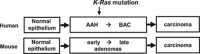
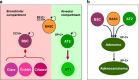
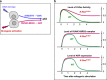
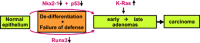
Lung adenocarcinomas, like other cancers, develop through the accumulation of epigenetic and genetic alterations. Numerous studies have shown that K-RAS mutation is among the most important early events in carcinogenesis of the lung. However, it is also well established that growth-stimulating signals feed back into growth-suppressing pathways, and any imbalance in these signaling networks will cause the cell to exit the cell cycle, thereby preventing uncontrolled cell growth. How, then, do K-RAS-activated cells evade cellular defense mechanisms? To answer this question, it is necessary to identify the molecular event(s) responsible for the development of early dysplastic lesions that are unable to defend against aberrant oncogene activation. Lineage-determining transcriptional regulators govern differentiation status during normal lung development, as well as in lung adenocarcinoma. Among the genes involved in K-RAS-induced lung tumorigenesis, RUNX3 is unique: inactivation of Runx3 in mouse lung induces lung adenoma and abrogates the ARF-p53 pathway. This observation raises the possibility of intimate cross-talk between the differentiation program and oncogene surveillance. In this review, we summarized evidences suggesting that K-RAS-activated cells do not evade cellular defense mechanisms per se; instead, cells with K-RAS mutations are selected only if they occur in cells in which defense mechanism is abrogated.
Figures
Similar articles
-
K-Ras-Activated Cells Can Develop into Lung Tumors When Runx3-Mediated Tumor Suppressor Pathways Are Abrogated.Mol Cells. 2020 Oct 31;43(10):889-897. doi: 10.14348/molcells.2020.0182. Mol Cells. 2020. PMID: 33115981 Free PMC article.
-
K-ras gene mutation enhances motility of immortalized airway cells and lung adenocarcinoma cells via Akt activation: possible contribution to non-invasive expansion of lung adenocarcinoma.Am J Pathol. 2004 Jan;164(1):91-100. doi: 10.1016/S0002-9440(10)63100-8. Am J Pathol. 2004. PMID: 14695323 Free PMC article.
-
Mutational and epigenetic evidence for independent pathways for lung adenocarcinomas arising in smokers and never smokers.Cancer Res. 2006 Feb 1;66(3):1371-5. doi: 10.1158/0008-5472.CAN-05-2625. Cancer Res. 2006. PMID: 16452191
-
Cigarette smoking and K-ras mutations in pancreas, lung and colorectal adenocarcinomas: etiopathogenic similarities, differences and paradoxes.Mutat Res. 2009 Sep-Dec;682(2-3):83-93. doi: 10.1016/j.mrrev.2009.07.003. Epub 2009 Aug 3. Mutat Res. 2009. PMID: 19651236 Review.
-
The ras oncogenes in human lung cancer.Am Rev Respir Dis. 1990 Dec;142(6 Pt 2):S27-30. doi: 10.1164/ajrccm/142.6_Pt_2.S27. Am Rev Respir Dis. 1990. PMID: 2252272 Review.
Cited by
-
HPGDS is a novel prognostic marker associated with lipid metabolism and aggressiveness in lung adenocarcinoma.Front Oncol. 2022 Oct 17;12:894485. doi: 10.3389/fonc.2022.894485. eCollection 2022. Front Oncol. 2022. PMID: 36324576 Free PMC article.
-
circLARP4 induces cellular senescence through regulating miR-761/RUNX3/p53/p21 signaling in hepatocellular carcinoma.Cancer Sci. 2019 Feb;110(2):568-581. doi: 10.1111/cas.13901. Epub 2019 Jan 4. Cancer Sci. 2019. PMID: 30520539 Free PMC article.
-
LIN28B enhanced tumorigenesis in an autochthonous KRASG12V-driven lung carcinoma mouse model.Oncogene. 2018 May;37(20):2746-2756. doi: 10.1038/s41388-018-0158-7. Epub 2018 Mar 5. Oncogene. 2018. PMID: 29503447
-
The Impact of Diet on the Involvement of Non-Coding RNAs, Extracellular Vesicles, and Gut Microbiome-Virome in Colorectal Cancer Initiation and Progression.Front Oncol. 2020 Dec 14;10:583372. doi: 10.3389/fonc.2020.583372. eCollection 2020. Front Oncol. 2020. PMID: 33381452 Free PMC article. Review.
-
Expression of PFKFB3 and Ki67 in lung adenocarcinomas and targeting PFKFB3 as a therapeutic strategy.Mol Cell Biochem. 2018 Aug;445(1-2):123-134. doi: 10.1007/s11010-017-3258-8. Epub 2018 Jan 11. Mol Cell Biochem. 2018. PMID: 29327288
References
-
- 1Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin 1999; 49: 8–31. - PubMed
-
- 2Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11–30. - PubMed
-
- 3Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell 2002; 1: 49–52. - PubMed
-
- 4Travis WD. Pathology of lung cancer. Clin Chest Med 2002; 23: 65–81. - PubMed
-
- 5Wistuba II, Gazdar AF. Lung cancer preneoplasia. Annu Rev Pathol 2006; 1: 331–348. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

