Aging increases microglial proliferation, delays cell migration, and decreases cortical neurogenesis after focal cerebral ischemia
- PMID: 25958332
- PMCID: PMC4437744
- DOI: 10.1186/s12974-015-0314-8
Aging increases microglial proliferation, delays cell migration, and decreases cortical neurogenesis after focal cerebral ischemia
Abstract
Background: Aging is not just a risk factor of stroke, but it has also been associated with poor recovery. It is known that stroke-induced neurogenesis is reduced but maintained in the aged brain. However, there is no consensus on how neurogenesis is affected after stroke in aged animals. Our objective is to determine the role of aging on the process of neurogenesis after stroke.
Methods: We have studied neurogenesis by analyzing proliferation, migration, and formation of new neurons, as well as inflammatory parameters, in a model of cerebral ischemia induced by permanent occlusion of the middle cerebral artery in young- (2 to 3 months) and middle-aged mice (13 to 14 months).
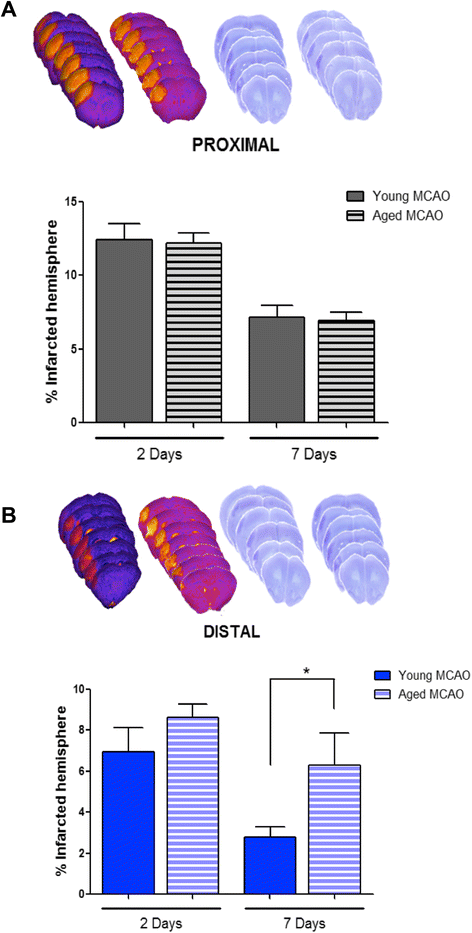
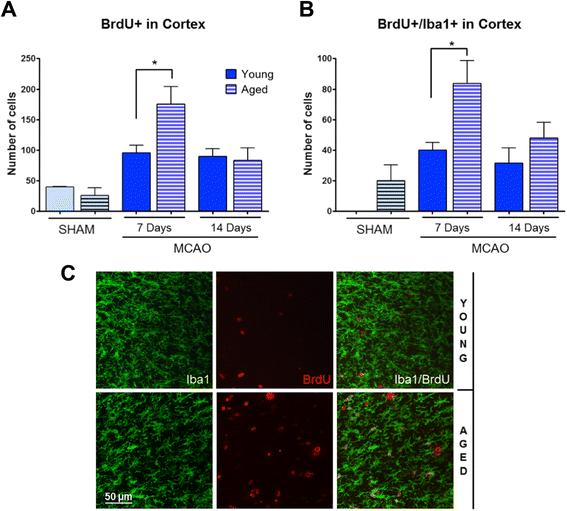
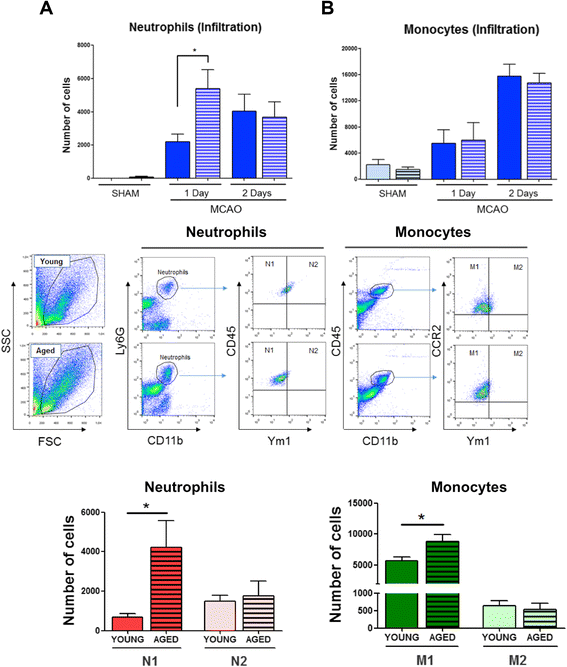
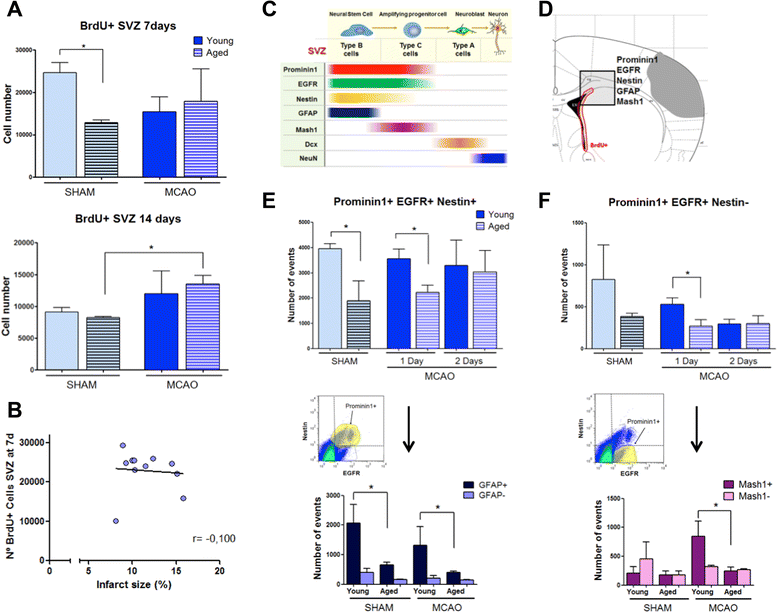
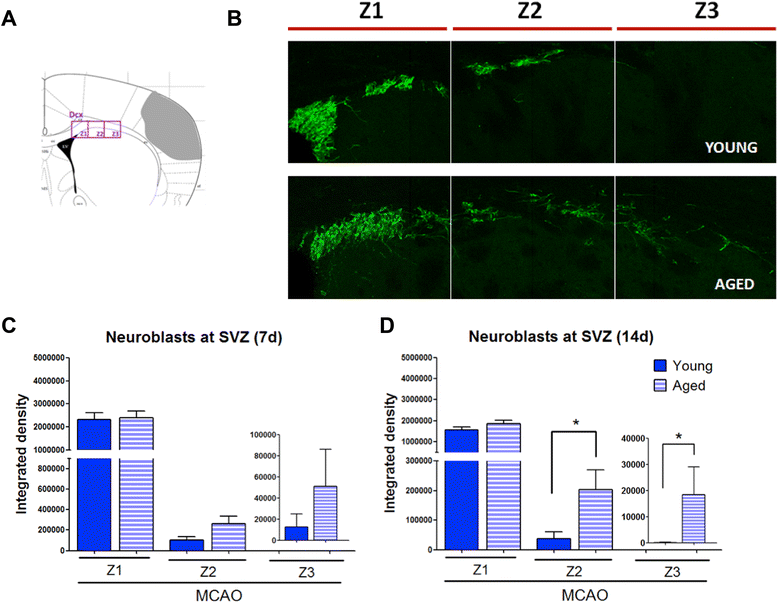
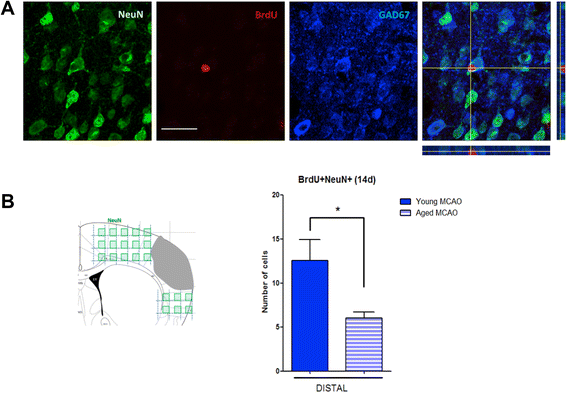
Results: Aging increased both microglial proliferation, as shown by a higher number of BrdU(+) cells and BrdU/Iba1(+) cells in the ischemic boundary and neutrophil infiltration. Interestingly, aging increased the number of M1 monocytes and N1 neutrophils, consistent with pro-inflammatory phenotypes when compared with the alternative M2 and N2 phenotypes. Aging also inhibited (subventricular zone) SVZ cell proliferation by decreasing both the number of astrocyte-like type-B (prominin-1(+)/epidermal growth factor receptor (EGFR)(+)/nestin(+)/glial fibrillary acidic protein (GFAP)(+) cells) and type-C cells (prominin-1(+)/EGFR(+)/nestin(-)/Mash1(+) cells), and not affecting apoptosis, 1 day after stroke. Aging also inhibited migration of neuroblasts (DCX(+) cells), as indicated by an accumulation of neuroblasts at migratory zones 14 days after injury; consistently, aged mice presented a smaller number of differentiated interneurons (NeuN(+)/BrdU(+) and GAD67(+) cells) in the peri-infarct cortical area 14 days after stroke.
Conclusions: Our data confirm that stroke-induced neurogenesis is maintained but reduced in aged animals. Importantly, we now demonstrate that aging not only inhibits proliferation of specific SVZ cell subtypes but also blocks migration of neuroblasts to the damaged area and decreases the number of new interneurons in the cortical peri-infarct area. Thus, our results highlight the importance of using aged animals for translation to clinical studies.
Figures
Similar articles
-
Toll-like receptor 4 modulates cell migration and cortical neurogenesis after focal cerebral ischemia.FASEB J. 2014 Nov;28(11):4710-8. doi: 10.1096/fj.14-252452. Epub 2014 Jul 25. FASEB J. 2014. PMID: 25063846
-
VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration.J Neurosci Res. 2007 Mar;85(4):740-7. doi: 10.1002/jnr.21169. J Neurosci Res. 2007. PMID: 17243175
-
Cilostazol attenuates ischemic brain injury and enhances neurogenesis in the subventricular zone of adult mice after transient focal cerebral ischemia.Neuroscience. 2010 Dec 29;171(4):1367-76. doi: 10.1016/j.neuroscience.2010.10.008. Epub 2010 Oct 8. Neuroscience. 2010. PMID: 20933581
-
Cellular and molecular determinants of stroke-induced changes in subventricular zone cell migration.Antioxid Redox Signal. 2011 May 15;14(10):1877-88. doi: 10.1089/ars.2010.3435. Epub 2010 Nov 1. Antioxid Redox Signal. 2011. PMID: 20673127 Free PMC article. Review.
-
Bioactive components of Chinese herbal medicine enhance endogenous neurogenesis in animal models of ischemic stroke: A systematic analysis.Medicine (Baltimore). 2016 Oct;95(40):e4904. doi: 10.1097/MD.0000000000004904. Medicine (Baltimore). 2016. PMID: 27749547 Free PMC article. Review.
Cited by
-
Neutrophil Heterogeneity and its Roles in the Inflammatory Network after Ischemic Stroke.Curr Neuropharmacol. 2023;21(3):621-650. doi: 10.2174/1570159X20666220706115957. Curr Neuropharmacol. 2023. PMID: 35794770 Free PMC article.
-
Determining the effect of aging, recovery time, and post-stroke memantine treatment on delayed thalamic gliosis after cortical infarct.Sci Rep. 2021 Jun 15;11(1):12613. doi: 10.1038/s41598-021-91998-3. Sci Rep. 2021. PMID: 34131204 Free PMC article.
-
Ischemic preconditioning induces cortical microglial proliferation and a transcriptomic program of robust cell cycle activation.Glia. 2020 Jan;68(1):76-94. doi: 10.1002/glia.23701. Epub 2019 Aug 17. Glia. 2020. PMID: 31420975 Free PMC article.
-
Non-monotonic Changes in Progenitor Cell Behavior and Gene Expression during Aging of the Adult V-SVZ Neural Stem Cell Niche.Stem Cell Reports. 2017 Dec 12;9(6):1931-1947. doi: 10.1016/j.stemcr.2017.10.005. Epub 2017 Nov 9. Stem Cell Reports. 2017. PMID: 29129683 Free PMC article.
-
Promoting Neurovascular Recovery in Aged Mice after Ischemic Stroke - Prophylactic Effect of Omega-3 Polyunsaturated Fatty Acids.Aging Dis. 2017 Oct 1;8(5):531-545. doi: 10.14336/AD.2017.0520. eCollection 2017 Oct. Aging Dis. 2017. PMID: 28966799 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

