Targeting glutamate uptake to treat alcohol use disorders
- PMID: 25954150
- PMCID: PMC4407613
- DOI: 10.3389/fnins.2015.00144
Targeting glutamate uptake to treat alcohol use disorders
Abstract
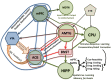
Alcoholism is a serious public health concern that is characterized by the development of tolerance to alcohol's effects, increased consumption, loss of control over drinking and the development of physical dependence. This cycle is often times punctuated by periods of abstinence, craving and relapse. The development of tolerance and the expression of withdrawal effects, which manifest as dependence, have been to a great extent attributed to neuroadaptations within the mesocorticolimbic and extended amygdala systems. Alcohol affects various neurotransmitter systems in the brain including the adrenergic, cholinergic, dopaminergic, GABAergic, glutamatergic, peptidergic, and serotonergic systems. Due to the myriad of neurotransmitter and neuromodulator systems affected by alcohol, the efficacies of current pharmacotherapies targeting alcohol dependence are limited. Importantly, research findings of changes in glutamatergic neurotransmission induced by alcohol self- or experimenter-administration have resulted in a focus on therapies targeting glutamatergic receptors and normalization of glutamatergic neurotransmission. Glutamatergic receptors implicated in the effects of ethanol include the ionotropic glutamate receptors (AMPA, Kainate, and NMDA) and some metabotropic glutamate receptors. Regarding glutamatergic homeostasis, ceftriaxone, MS-153, and GPI-1046, which upregulate glutamate transporter 1 (GLT1) expression in mesocorticolimbic brain regions, reduce alcohol intake in genetic animal models of alcoholism. Given the hyperglutamatergic/hyperexcitable state of the central nervous system induced by chronic alcohol abuse and withdrawal, the evidence thus far indicates that a restoration of glutamatergic concentrations and activity within the mesocorticolimbic system and extended amygdala as well as multiple memory systems holds great promise for the treatment of alcohol dependence.
Keywords: EAAT2; GLT1; alcohol; dopamine; glutamate; neurotransmitter.
Figures
Similar articles
-
Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism.Neurochem Int. 1995 Apr;26(4):305-36; discussion 337-42. doi: 10.1016/0197-0186(94)00139-l. Neurochem Int. 1995. PMID: 7633325 Review.
-
Glutamatergic targets for new alcohol medications.Psychopharmacology (Berl). 2013 Oct;229(3):539-54. doi: 10.1007/s00213-013-3226-2. Epub 2013 Sep 1. Psychopharmacology (Berl). 2013. PMID: 23995381 Free PMC article. Review.
-
Role of glutamatergic system and mesocorticolimbic circuits in alcohol dependence.Prog Neurobiol. 2018 Dec;171:32-49. doi: 10.1016/j.pneurobio.2018.10.001. Epub 2018 Oct 11. Prog Neurobiol. 2018. PMID: 30316901 Free PMC article. Review.
-
Metabotropic and ionotropic glutamate receptors as potential targets for the treatment of alcohol use disorder.Neurosci Biobehav Rev. 2017 Jun;77:14-31. doi: 10.1016/j.neubiorev.2017.02.024. Epub 2017 Feb 24. Neurosci Biobehav Rev. 2017. PMID: 28242339 Free PMC article. Review.
-
Glutamatergic plasticity and alcohol dependence-induced alterations in reward, affect and cognition.Prog Neuropsychopharmacol Biol Psychiatry. 2016 Feb 4;65:309-20. doi: 10.1016/j.pnpbp.2015.08.012. Epub 2015 Sep 1. Prog Neuropsychopharmacol Biol Psychiatry. 2016. PMID: 26341050 Free PMC article. Review.
Cited by
-
Transcranial Direct Current Stimulation (tDCS) Induces Analgesia in Rats with Neuropathic Pain and Alcohol Abstinence.Neurochem Res. 2020 Nov;45(11):2653-2663. doi: 10.1007/s11064-020-03116-w. Epub 2020 Aug 25. Neurochem Res. 2020. PMID: 32840761
-
Alcohol Use Disorder, Neurodegeneration, Alzheimer's and Parkinson's Disease: Interplay Between Oxidative Stress, Neuroimmune Response and Excitotoxicity.Front Cell Neurosci. 2020 Aug 31;14:282. doi: 10.3389/fncel.2020.00282. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33061892 Free PMC article. Review.
-
Amoxicillin and amoxicillin/clavulanate reduce ethanol intake and increase GLT-1 expression as well as AKT phosphorylation in mesocorticolimbic regions.Brain Res. 2015 Oct 5;1622:397-408. doi: 10.1016/j.brainres.2015.07.008. Epub 2015 Jul 10. Brain Res. 2015. PMID: 26168897 Free PMC article.
-
SNARE Complex-Associated Proteins and Alcohol.Alcohol Clin Exp Res. 2020 Jan;44(1):7-18. doi: 10.1111/acer.14238. Epub 2019 Dec 11. Alcohol Clin Exp Res. 2020. PMID: 31724225 Free PMC article. Review.
-
Effects of Ethanol Exposure on the Neurochemical Profile of a Transgenic Mouse Model with Enhanced Glutamate Release Using In Vivo 1H MRS.Neurochem Res. 2019 Jan;44(1):133-146. doi: 10.1007/s11064-018-2658-9. Epub 2018 Oct 17. Neurochem Res. 2019. PMID: 30334175 Free PMC article.
References
-
- Aal-Aaboda M., Alhaddad H., Osowik F., Nauli S. M., Sari Y. (2015). Effects of (R)-(-)-5-methyl-1-nicotinoyl-2-pyrazoline on glutamate transporter 1 and cysteine/glutamate exchanger as well as ethanol drinking behavior in male, alcohol-preferring rats. J. Neurosci. Res. 93, 930–937. 10.1002/jnr.23554 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

