K1 and K15 of Kaposi's Sarcoma-Associated Herpesvirus Are Partial Functional Homologues of Latent Membrane Protein 2A of Epstein-Barr Virus
- PMID: 25948739
- PMCID: PMC4473585
- DOI: 10.1128/JVI.00839-15
K1 and K15 of Kaposi's Sarcoma-Associated Herpesvirus Are Partial Functional Homologues of Latent Membrane Protein 2A of Epstein-Barr Virus
Abstract
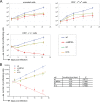
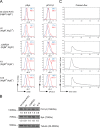
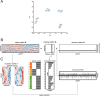
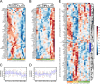
The human herpesviruses Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV) are associated with Hodgkin's lymphoma (HL) and Primary effusion lymphomas (PEL), respectively, which are B cell malignancies that originate from germinal center B cells. PEL cells but also a quarter of EBV-positive HL tumor cells do not express the genuine B cell receptor (BCR), a situation incompatible with survival of normal B cells. EBV encodes LMP2A, one of EBV's viral latent membrane proteins, which likely replaces the BCR's survival signaling in HL. Whether KSHV encodes a viral BCR mimic that contributes to oncogenesis is not known because an experimental model of KSHV-mediated B cell transformation is lacking. We addressed this uncertainty with mutant EBVs encoding the KSHV genes K1 or K15 in lieu of LMP2A and infected primary BCR-negative (BCR(-)) human B cells with them. We confirmed that the survival of BCR(-) B cells and their proliferation depended on an active LMP2A signal. Like LMP2A, the expression of K1 and K15 led to the survival of BCR(-) B cells prone to apoptosis, supported their proliferation, and regulated a similar set of cellular target genes. K1 and K15 encoded proteins appear to have noncomplementing, redundant functions in this model, but our findings suggest that both KSHV proteins can replace LMP2A's key activities contributing to the survival, activation and proliferation of BCR(-) PEL cells in vivo.
Importance: Several herpesviruses encode oncogenes that are receptor-like proteins. Often, they are constitutively active providing important functions to the latently infected cells. LMP2A of Epstein-Barr virus (EBV) is such a receptor that mimics an activated B cell receptor, BCR. K1 and K15, related receptors of Kaposi's sarcoma-associated herpesvirus (KSHV) expressed in virus-associated tumors, have less obvious functions. We found in infection experiments that both viral receptors of KSHV can replace LMP2A and deliver functions similar to the endogenous BCR. K1, K15, and LMP2A also control the expression of a related set of cellular genes in primary human B cells, the target cells of EBV and KSHV. The observed phenotypes, as well as the known characteristics of these genes, argue for their contributions to cellular survival, B cell activation, and proliferation. Our findings provide one possible explanation for the tumorigenicity of KSHV, which poses a severe problem in immunocompromised patients.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
The M type K15 protein of Kaposi's sarcoma-associated herpesvirus regulates microRNA expression via its SH2-binding motif to induce cell migration and invasion.J Virol. 2009 Jan;83(2):622-32. doi: 10.1128/JVI.00869-08. Epub 2008 Oct 29. J Virol. 2009. PMID: 18971265 Free PMC article.
-
Suppression of the LMP2A target gene, EGR-1, protects Hodgkin's lymphoma cells from entry to the EBV lytic cycle.J Pathol. 2013 Aug;230(4):399-409. doi: 10.1002/path.4198. J Pathol. 2013. PMID: 23592216
-
Distinct patterns of viral antigen expression in Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus coinfected body-cavity-based lymphoma cell lines: potential switches in latent gene expression due to coinfection.Virology. 1999 Sep 15;262(1):18-30. doi: 10.1006/viro.1999.9876. Virology. 1999. PMID: 10489337
-
Ubiquitin-Mediated Effects on Oncogenesis during EBV and KSHV Infection.Viruses. 2024 Sep 26;16(10):1523. doi: 10.3390/v16101523. Viruses. 2024. PMID: 39459858 Free PMC article. Review.
-
Pathological Features of Kaposi's Sarcoma-Associated Herpesvirus Infection.Adv Exp Med Biol. 2018;1045:357-376. doi: 10.1007/978-981-10-7230-7_16. Adv Exp Med Biol. 2018. PMID: 29896675 Review.
Cited by
-
Contribution of the KSHV and EBV lytic cycles to tumourigenesis.Curr Opin Virol. 2018 Oct;32:60-70. doi: 10.1016/j.coviro.2018.08.014. Epub 2018 Sep 28. Curr Opin Virol. 2018. PMID: 30268927 Free PMC article. Review.
-
Pomalidomide increases immune surface marker expression and immune recognition of oncovirus-infected cells.Oncoimmunology. 2018 Dec 5;8(2):e1546544. doi: 10.1080/2162402X.2018.1546544. eCollection 2019. Oncoimmunology. 2018. PMID: 30713808 Free PMC article.
-
K15 Protein of Kaposi's Sarcoma Herpesviruses Increases Endothelial Cell Proliferation and Migration through Store-Operated Calcium Entry.Viruses. 2018 May 24;10(6):282. doi: 10.3390/v10060282. Viruses. 2018. PMID: 29795033 Free PMC article.
-
Expression and Subcellular Localization of the Kaposi's Sarcoma-Associated Herpesvirus K15P Protein during Latency and Lytic Reactivation in Primary Effusion Lymphoma Cells.J Virol. 2017 Oct 13;91(21):e01370-17. doi: 10.1128/JVI.01370-17. Print 2017 Nov 1. J Virol. 2017. PMID: 28835496 Free PMC article.
-
First Days in the Life of Naive Human B Lymphocytes Infected with Epstein-Barr Virus.mBio. 2019 Sep 17;10(5):e01723-19. doi: 10.1128/mBio.01723-19. mBio. 2019. PMID: 31530670 Free PMC article.
References
-
- Beaufils P, Choquet D, Mamoun RZ, Malissen B. 1993. The (YXXL/I)2 signalling motif found in the cytoplasmic segments of the bovine leukaemia virus envelope protein and Epstein-Barr virus latent membrane protein 2A can elicit early and late lymphocyte activation events. EMBO J 12:5105–5112. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

