Cellular Responses Modulated by FGF-2 Adsorbed on Albumin/Heparin Layer-by-Layer Assemblies
- PMID: 25945799
- PMCID: PMC4422587
- DOI: 10.1371/journal.pone.0125484
Cellular Responses Modulated by FGF-2 Adsorbed on Albumin/Heparin Layer-by-Layer Assemblies
Abstract
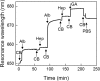
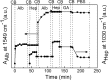
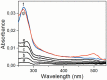
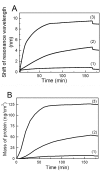
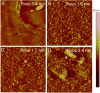
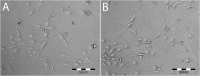
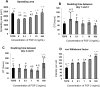
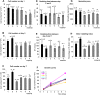
In a typical cell culture system, growth factors immobilized on the cell culture surfaces can serve as a reservoir of bio-signaling molecules, without the need to supplement them additionally into the culture medium. In this paper, we report on the fabrication of albumin/heparin (Alb/Hep) assemblies for controlled binding of basic fibroblast growth factor (FGF-2). The surfaces were constructed by layer-by-layer adsorption of polyelectrolytes albumin and heparin and were subsequently stabilized by covalent crosslinking with glutaraldehyde. An analysis of the surface morphology by atomic force microscopy showed that two Alb/Hep bilayers are required to cover the surface of substrate. The formation of the Alb/Hep assemblies was monitored by the surface plasmon resonance (SPR), the infrared multiinternal reflection spectroscopy (FTIR MIRS) and UV/VIS spectroscopy. The adsorption of FGF-2 on the cross-linked Alb/Hep was followed by SPR. The results revealed that FGF-2 binds to the Alb/Hep assembly in a dose and time-dependent manner up to the surface concentration of 120 ng/cm(2). The bioactivity of the adsorbed FGF-2 was assessed in experiments in vitro, using calf pulmonary arterial endothelial cells (CPAE). CPAE cells could attach and proliferate on Alb/Hep surfaces. The adsorbed FGF-2 was bioactive and stimulated both the proliferation and the differentiation of CPAE cells. The improvement was more pronounced at a lower FGF-2 surface concentration (30 ng/cm(2)) than on surfaces with a higher concentration of FGF-2 (120 ng/cm(2)).
Conflict of interest statement
Figures

Similar articles
-
Polysaccharide-based polyelectrolyte multilayer surface coatings can enhance mesenchymal stem cell response to adsorbed growth factors.Biomacromolecules. 2010 Oct 11;11(10):2629-39. doi: 10.1021/bm1005799. Biomacromolecules. 2010. PMID: 20795698
-
Switching of cell growth/detachment on heparin-functionalized thermoresponsive surface for rapid cell sheet fabrication and manipulation.Biomaterials. 2013 Jun;34(17):4214-22. doi: 10.1016/j.biomaterials.2013.02.056. Epub 2013 Mar 13. Biomaterials. 2013. PMID: 23498894
-
Surface immobilized protein multilayers for cell seeding.Langmuir. 2005 Aug 16;21(17):7877-83. doi: 10.1021/la046846o. Langmuir. 2005. PMID: 16089395
-
Preparation and preliminary in vitro evaluation of a bFGF-releasing heparin-conjugated poly(ε-caprolactone) membrane for guided bone regeneration.J Biomater Sci Polym Ed. 2015;26(10):600-16. doi: 10.1080/09205063.2015.1049044. Epub 2015 Jun 12. J Biomater Sci Polym Ed. 2015. PMID: 26065539
-
The role of basic fibroblast growth factor in glioblastoma multiforme and glioblastoma stem cells and in their in vitro culture.Cancer Lett. 2014 Apr 28;346(1):1-5. doi: 10.1016/j.canlet.2013.12.003. Epub 2013 Dec 11. Cancer Lett. 2014. PMID: 24333730 Review.
Cited by
-
Versatile Mitogenic and Differentiation-Inducible Layer Formation by Underwater Adhesive Polypeptides.Adv Sci (Weinh). 2021 Aug;8(16):e2100961. doi: 10.1002/advs.202100961. Epub 2021 Jun 26. Adv Sci (Weinh). 2021. PMID: 34174166 Free PMC article.
-
Growth Factors VEGF-A165 and FGF-2 as Multifunctional Biomolecules Governing Cell Adhesion and Proliferation.Int J Mol Sci. 2021 Feb 12;22(4):1843. doi: 10.3390/ijms22041843. Int J Mol Sci. 2021. PMID: 33673317 Free PMC article.
-
Immobilization of Growth Factors for Cell Therapy Manufacturing.Front Bioeng Biotechnol. 2020 Jun 19;8:620. doi: 10.3389/fbioe.2020.00620. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32637403 Free PMC article. Review.
-
Improvements on biological and antimicrobial properties of titanium modified by AgNPs-loaded chitosan-heparin polyelectrolyte multilayers.J Mater Sci Mater Med. 2019 Apr 23;30(5):52. doi: 10.1007/s10856-019-6250-x. J Mater Sci Mater Med. 2019. PMID: 31016469
-
Growth Factor Immobilization to Synthetic Hydrogels: Bioactive bFGF-Functionalized Polyisocyanide Hydrogels.Adv Healthc Mater. 2023 Oct;12(27):e2301109. doi: 10.1002/adhm.202301109. Epub 2023 Aug 10. Adv Healthc Mater. 2023. PMID: 37526214 Free PMC article.
References
-
- Ito Y, Zheng J, Imanishi Y. Immobilization of Biosignal Proteins to Control Cellular Functions In: Ogata N, Kim S, Feijen J, Okano T, editors. Advanced Biomaterials in Biomedical Engineering and Drug Delivery Systems: Springer Japan; 1996. pp. 265–266.
-
- Cha T, Guo A, Zhu XY. Enzymatic activity on a chip: the critical role of protein orientation. Proteomics. 2005;5: 416–419. - PubMed
-
- Butler JE. Solid supports in enzyme-linked immunosorbent assay and other solid-phase immunoassays. Methods. 2000;22: 4–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

