Characterization and applications of Nanobodies against human procalcitonin selected from a novel naïve Nanobody phage display library
- PMID: 25944262
- PMCID: PMC4475299
- DOI: 10.1186/s12951-015-0091-7
Characterization and applications of Nanobodies against human procalcitonin selected from a novel naïve Nanobody phage display library
Abstract
Background: Nanobodies (Nbs) are single-domain antigen-binding fragments derived from the camelids heavy-chain only antibodies (HCAbs). Their unique advantageous properties make Nbs highly attractive in various applications. The general approach to obtain Nbs is to isolate them from immune libraries by phage display technology. However, it is unfeasible when the antigens are toxic, lethal, transmissible or of low immunogenicity. Naïve libraries could be an alternative way to solve the above problems.
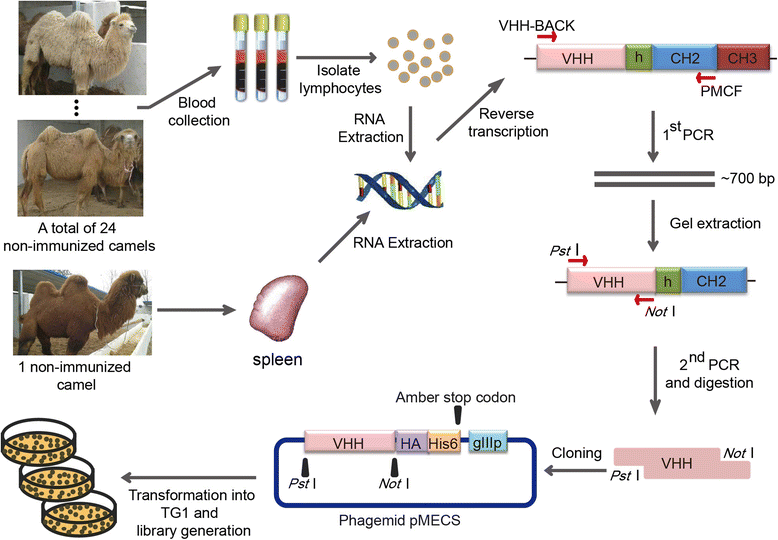
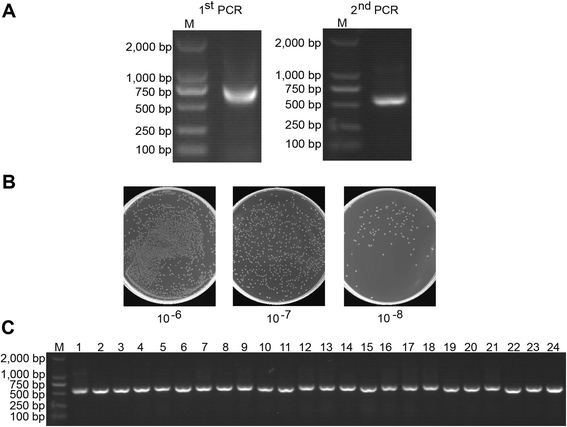
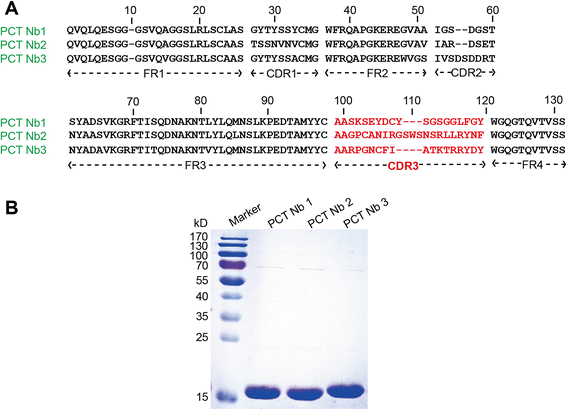
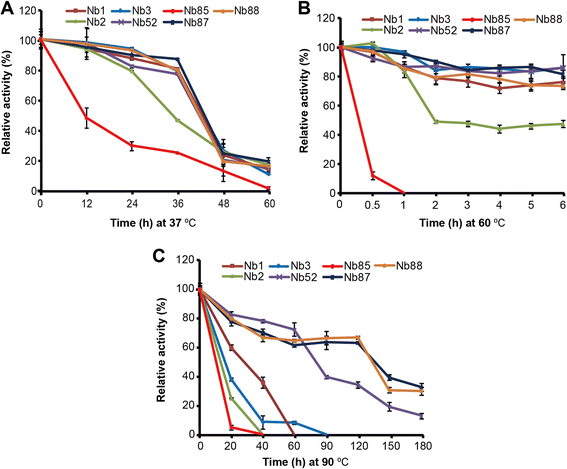
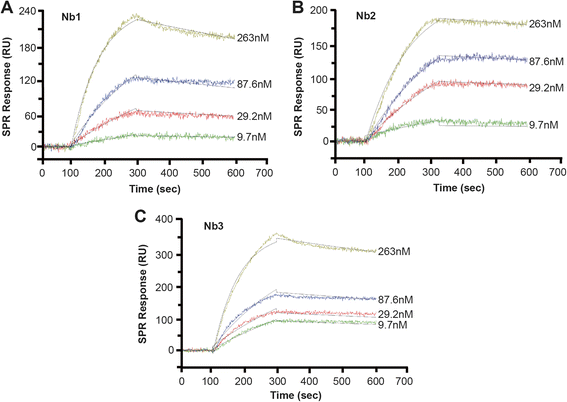
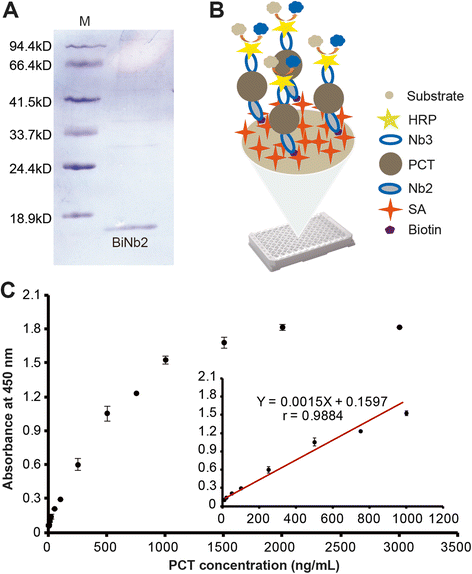
Results: We constructed a large camel naïve phage display Nanobody (Nb) library with great diversity. The generated library contains to 6.86 × 10(11) clones and to our best of knowledge, this is the biggest naïve phage display Nb library. Then Nbs against human procalcitonin (PCT) were isolated from this library. These Nbs showed comparable affinity and antigen-binding thermostability at 37°C and 60°C compared to the PCT Nbs from an immune phage-displayed library. Furthermore, two PCT Nbs that recognize unique epitopes on PCT have been successfully applied to develop a sandwich enzyme-linked immunosorbent assay (ELISA) to detect PCT, which showed a linear working range from 10-1000 ng/mL of PCT.
Conclusion: We have constructed a large and diverse naïve phage display Nb library, which potentially functioning as a good resource for selecting antigen-binders with high quality. Moreover, functional Nbs against PCT were successfully characterized and applied, providing great values on medical application.
Figures
Similar articles
-
Construction of a synthetic phage-displayed Nanobody library with CDR3 regions randomized by trinucleotide cassettes for diagnostic applications.J Transl Med. 2014 Dec 10;12:343. doi: 10.1186/s12967-014-0343-6. J Transl Med. 2014. PMID: 25496223 Free PMC article.
-
Bactrian camel nanobody-based immunoassay for specific and sensitive detection of Cry1Fa toxin.Toxicon. 2014 Dec 15;92:186-92. doi: 10.1016/j.toxicon.2014.10.024. Epub 2014 Oct 30. Toxicon. 2014. PMID: 25448390
-
Generation and characterization of nanobodies against rhGH expressed as sfGFP fusion protein.Gen Comp Endocrinol. 2014 Aug 1;204:33-42. doi: 10.1016/j.ygcen.2014.05.018. Epub 2014 May 22. Gen Comp Endocrinol. 2014. PMID: 24859761
-
Identification of Useful Nanobodies by Phage Display of Immune Single Domain Libraries Derived from Camelid Heavy Chain Antibodies.Curr Pharm Des. 2016;22(43):6500-6518. doi: 10.2174/1381612822666160923114417. Curr Pharm Des. 2016. PMID: 27669966 Review.
-
Recent advances in the selection and identification of antigen-specific nanobodies.Mol Immunol. 2018 Apr;96:37-47. doi: 10.1016/j.molimm.2018.02.012. Epub 2018 Feb 22. Mol Immunol. 2018. PMID: 29477934 Review.
Cited by
-
Nanobodies targeting immune checkpoint molecules for tumor immunotherapy and immunoimaging (Review).Int J Mol Med. 2021 Feb;47(2):444-454. doi: 10.3892/ijmm.2020.4817. Epub 2020 Dec 14. Int J Mol Med. 2021. PMID: 33416134 Free PMC article. Review.
-
An Inside Job: Applications of Intracellular Single Domain Antibodies.Biomolecules. 2020 Dec 12;10(12):1663. doi: 10.3390/biom10121663. Biomolecules. 2020. PMID: 33322697 Free PMC article. Review.
-
Research progress and applications of nanobody in human infectious diseases.Front Pharmacol. 2022 Aug 12;13:963978. doi: 10.3389/fphar.2022.963978. eCollection 2022. Front Pharmacol. 2022. PMID: 36034845 Free PMC article. Review.
-
Preclinical development of a novel CD47 nanobody with less toxicity and enhanced anti-cancer therapeutic potential.J Nanobiotechnology. 2020 Jan 13;18(1):12. doi: 10.1186/s12951-020-0571-2. J Nanobiotechnology. 2020. PMID: 31931812 Free PMC article.
-
A guide to: generation and design of nanobodies.FEBS J. 2021 Apr;288(7):2084-2102. doi: 10.1111/febs.15515. Epub 2020 Aug 28. FEBS J. 2021. PMID: 32780549 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

