Dimethyl fumarate and the oleanane triterpenoids, CDDO-imidazolide and CDDO-methyl ester, both activate the Nrf2 pathway but have opposite effects in the A/J model of lung carcinogenesis
- PMID: 25939751
- PMCID: PMC4572919
- DOI: 10.1093/carcin/bgv061
Dimethyl fumarate and the oleanane triterpenoids, CDDO-imidazolide and CDDO-methyl ester, both activate the Nrf2 pathway but have opposite effects in the A/J model of lung carcinogenesis
Abstract
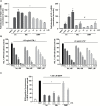
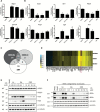
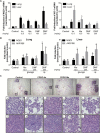
Lung cancer accounts for the highest number of cancer-related deaths in the USA, highlighting the need for better prevention and therapy. Activation of the Nrf2 pathway detoxifies harmful insults and reduces oxidative stress, thus preventing carcinogenesis in various preclinical models. However, constitutive activation of the Nrf2 pathway has been detected in numerous cancers, which confers a survival advantage to tumor cells and a poor prognosis. In our study, we compared the effects of two clinically relevant classes of Nrf2 activators, dimethyl fumarate (DMF) and the synthetic oleanane triterpenoids, CDDO-imidazolide (CDDO-Im) and CDDO-methyl ester (CDDO-Me) in RAW 264.7 mouse macrophage-like cells, in VC1 lung cancer cells and in the A/J model of lung cancer. Although the triterpenoids and DMF both activated the Nrf2 pathway, CDDO-Im and CDDO-Me were markedly more potent than DMF. All of these drugs reduced the production of reactive oxygen species and inhibited nitric oxide production in RAW264.7 cells, but the triterpenoids were 100 times more potent than DMF in these assays. Microarray analysis revealed that only 52 of 99 Nrf2-target genes were induced by all three compounds, and each drug regulated a unique subset of Nrf2 genes. These drugs also altered the expression of other genes important in lung cancer independent of Nrf2. Although all three compounds enhanced the phosphorylation of CREB, only DMF increased the phosphorylation of Akt. CDDO-Me, at either 12.5 or 50mg/kg of diet, was the most effective drug in our lung cancer mouse model. Specifically, CDDO-Me significantly reduced the average tumor number, size and burden compared with the control group (P < 0.05). Additionally, 52% of the tumors in the control group were high-grade tumors compared with only 14% in the CDDO-Me group. Though less potent, CDDO-Im had similar activity as CDDO-Me. In contrast, 61-63% of the tumors in the DMF groups (400-1200mg/kg diet) were high-grade tumors compared with 52% for the controls (P < 0.05). Additionally, DMF significantly increased the average number of tumors compared with the controls (P < 0.05). Thus, in contrast to the triterpenoids, which effectively reduced pathogenesis in A/J mice, DMF enhanced the severity of lung carcinogenesis in these mice. Collectively, these results suggest that although CDDO-Im, CDDO-Me and DMF all activate the Nrf2 pathway, they target distinct genes and signaling pathways, resulting in opposite effects for the prevention of experimental lung cancer.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


Similar articles
-
Preclinical evaluation of targeting the Nrf2 pathway by triterpenoids (CDDO-Im and CDDO-Me) for protection from LPS-induced inflammatory response and reactive oxygen species in human peripheral blood mononuclear cells and neutrophils.Antioxid Redox Signal. 2007 Nov;9(11):1963-70. doi: 10.1089/ars.2007.1745. Antioxid Redox Signal. 2007. PMID: 17822364 Free PMC article.
-
The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling.Cancer Res. 2005 Jun 1;65(11):4789-98. doi: 10.1158/0008-5472.CAN-04-4539. Cancer Res. 2005. PMID: 15930299
-
Novel synthetic pyridyl analogues of CDDO-Imidazolide are useful new tools in cancer prevention.Pharmacol Res. 2015 Oct;100:135-47. doi: 10.1016/j.phrs.2015.07.024. Epub 2015 Jul 31. Pharmacol Res. 2015. PMID: 26238177
-
Synthesis and Anticancer Activity of CDDO and CDDO-Me, Two Derivatives of Natural Triterpenoids.Molecules. 2019 Nov 13;24(22):4097. doi: 10.3390/molecules24224097. Molecules. 2019. PMID: 31766211 Free PMC article. Review.
-
Preclinical evidences toward the use of triterpenoid CDDO-Me for solid cancer prevention and treatment.Mol Cancer. 2014 Feb 20;13:30. doi: 10.1186/1476-4598-13-30. Mol Cancer. 2014. PMID: 24552536 Free PMC article. Review.
Cited by
-
CDDO-Me, Sulforaphane and tBHQ attenuate the RANKL-induced osteoclast differentiation via activating the NRF2-mediated antioxidant response.Biochem Biophys Res Commun. 2019 Apr 9;511(3):637-643. doi: 10.1016/j.bbrc.2019.02.095. Epub 2019 Feb 27. Biochem Biophys Res Commun. 2019. PMID: 30826055 Free PMC article.
-
NF-E2-Related Factor 2 Regulates Interferon Receptor Expression and Alters Macrophage Polarization in Lupus.Arthritis Rheumatol. 2020 Oct;72(10):1707-1720. doi: 10.1002/art.41383. Epub 2020 Aug 9. Arthritis Rheumatol. 2020. PMID: 32500632 Free PMC article.
-
Nuclear Factor Erythroid 2-Related Factor 2 in Regulating Cancer Metabolism.Antioxid Redox Signal. 2020 Nov 1;33(13):966-997. doi: 10.1089/ars.2020.8024. Epub 2020 Mar 18. Antioxid Redox Signal. 2020. PMID: 31989830 Free PMC article. Review.
-
New Insights into the Role of Oxidative Stress Mechanisms in the Pathophysiology and Treatment of Multiple Sclerosis.Oxid Med Cell Longev. 2016;2016:1973834. doi: 10.1155/2016/1973834. Epub 2016 Oct 18. Oxid Med Cell Longev. 2016. PMID: 27829982 Free PMC article. Review.
-
Contribution of Nrf2 Modulation to the Mechanism of Action of Analgesic and Anti-inflammatory Drugs in Pre-clinical and Clinical Stages.Front Pharmacol. 2019 Jan 11;9:1536. doi: 10.3389/fphar.2018.01536. eCollection 2018. Front Pharmacol. 2019. PMID: 30687097 Free PMC article. Review.
References
-
- Siegel R., et al. (2014) Cancer statistics, 2014. CA Cancer J. Clin., 64, 9–29. - PubMed
-
- Suzuki T., et al. (2013) Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol. Sci., 34, 340–346. - PubMed
-
- Hayes J.D., et al. (2010) Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid. Redox Signal., 13, 1713–1748. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

