Asymmetric synthesis and in vitro and in vivo activity of tetrahydroquinolines featuring a diverse set of polar substitutions at the 6 position as mixed-efficacy μ opioid receptor/δ opioid receptor ligands
- PMID: 25938166
- PMCID: PMC4676711
- DOI: 10.1021/acschemneuro.5b00100
Asymmetric synthesis and in vitro and in vivo activity of tetrahydroquinolines featuring a diverse set of polar substitutions at the 6 position as mixed-efficacy μ opioid receptor/δ opioid receptor ligands
Abstract
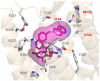
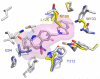
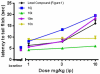
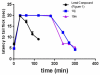
We previously reported a small series of mixed-efficacy μ opioid receptor (MOR) agonist/δ opioid receptor (DOR) antagonist peptidomimetics featuring a tetrahydroquinoline scaffold and showed the promise of this series as effective analgesics after intraperitoneal administration in mice. We report here an expanded structure-activity relationship study of the pendant region of these compounds and focus in particular on the incorporation of heteroatoms into this side chain. These analogues provide new insight into the binding requirements for this scaffold at MOR, DOR, and the κ opioid receptor (KOR), and several of them (10j, 10k, 10m, and 10n) significantly improve upon the overall MOR agonist/DOR antagonist profile of our previous compounds. In vivo data for 10j, 10k, 10m, and 10n are also reported and show the antinociceptive potency and duration of action of compounds 10j and 10m to be comparable to those of morphine.
Keywords: Opioid; dependence; intraperitoneal; mixed efficacy; tetrahydroquinoline; tolerance.
Figures
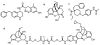
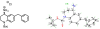
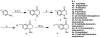
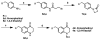
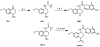
Similar articles
-
Effects of N-Substitutions on the Tetrahydroquinoline (THQ) Core of Mixed-Efficacy μ-Opioid Receptor (MOR)/δ-Opioid Receptor (DOR) Ligands.J Med Chem. 2016 May 26;59(10):4985-98. doi: 10.1021/acs.jmedchem.6b00308. Epub 2016 May 16. J Med Chem. 2016. PMID: 27148755 Free PMC article.
-
Synthesis and Pharmacological Evaluation of Novel C-8 Substituted Tetrahydroquinolines as Balanced-Affinity Mu/Delta Opioid Ligands for the Treatment of Pain.ACS Chem Neurosci. 2018 Jul 18;9(7):1840-1848. doi: 10.1021/acschemneuro.8b00139. Epub 2018 May 2. ACS Chem Neurosci. 2018. PMID: 29677442 Free PMC article.
-
Synthesis, modeling, and pharmacological evaluation of UMB 425, a mixed μ agonist/δ antagonist opioid analgesic with reduced tolerance liabilities.ACS Chem Neurosci. 2013 Sep 18;4(9):1256-66. doi: 10.1021/cn4000428. Epub 2013 Jun 11. ACS Chem Neurosci. 2013. PMID: 23713721 Free PMC article.
-
The search for opioid analgesics with limited tolerance liability.Peptides. 2020 Aug;130:170331. doi: 10.1016/j.peptides.2020.170331. Epub 2020 Jun 1. Peptides. 2020. PMID: 32497566 Review.
-
Opioid ligands with mixed mu/delta opioid receptor interactions: an emerging approach to novel analgesics.AAPS J. 2006 Mar 10;8(1):E118-25. doi: 10.1208/aapsj080114. AAPS J. 2006. PMID: 16584118 Free PMC article. Review.
Cited by
-
Peptidomimetics and Their Applications for Opioid Peptide Drug Discovery.Biomolecules. 2022 Sep 5;12(9):1241. doi: 10.3390/biom12091241. Biomolecules. 2022. PMID: 36139079 Free PMC article. Review.
-
The behavioral effects of a mixed efficacy antinociceptive peptide, VRP26, following chronic administration in mice.Psychopharmacology (Berl). 2016 Jul;233(13):2479-87. doi: 10.1007/s00213-016-4296-8. Epub 2016 Apr 27. Psychopharmacology (Berl). 2016. PMID: 27117141 Free PMC article.
-
Dual Pharmacophores Explored via Structure-Activity Relationship (SAR) Matrix: Insights into Potent, Bifunctional Opioid Ligand Design.J Med Chem. 2019 Apr 25;62(8):4193-4203. doi: 10.1021/acs.jmedchem.9b00378. Epub 2019 Apr 9. J Med Chem. 2019. PMID: 30916966 Free PMC article.
-
Rapid Synthesis of Boc-2',6'-dimethyl-l-tyrosine and Derivatives and Incorporation into Opioid Peptidomimetics.ACS Med Chem Lett. 2015 Oct 19;6(12):1199-203. doi: 10.1021/acsmedchemlett.5b00344. eCollection 2015 Dec 10. ACS Med Chem Lett. 2015. PMID: 26713104 Free PMC article.
-
Effects of N-Substitutions on the Tetrahydroquinoline (THQ) Core of Mixed-Efficacy μ-Opioid Receptor (MOR)/δ-Opioid Receptor (DOR) Ligands.J Med Chem. 2016 May 26;59(10):4985-98. doi: 10.1021/acs.jmedchem.6b00308. Epub 2016 May 16. J Med Chem. 2016. PMID: 27148755 Free PMC article.
References
-
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid Complications and Side Effects. Pain Physician. 2008;11:S105–120. - PubMed
-
- Hepburn MJ, Little PJ, Gingras J, Kuhn CM. Differential Effects of Naltrindole on Morphine-Induced Tolerance and Physical Dependence in Rats. J. Pharm. Exp. Ther. 1997;281:1350–1356. - PubMed
-
- Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective Blockage of Delta Opioid Receptors Prevents the Development of Morphine Tolerance and Dependence in Mice. J. Pharm. Exp. Ther. 1991;258:299–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

