Lowering the dietary omega-6: omega-3 does not hinder nonalcoholic fatty-liver disease development in a murine model
- PMID: 25934114
- PMCID: PMC4451001
- DOI: 10.1016/j.nutres.2015.04.003
Lowering the dietary omega-6: omega-3 does not hinder nonalcoholic fatty-liver disease development in a murine model
Abstract
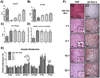
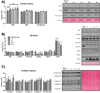
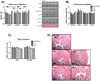
It is hypothesized that a high dietary n-6:n-3 (eg, 10-20:1) is partly responsible for the rise in obesity and related health ailments. However, no tightly controlled studies using high-fat diets differing in the n-6:n-3 have tested this hypothesis. The aim of the study was to determine the role that the dietary n-6:n-3 plays in non-alcoholic fatty-liver disease (NAFLD) and colitis development. We hypothesized that reducing the dietary n-6:n-3 would hinder the development of NAFLD and colitis. Male C57BL/6 J mice were fed high-fat diets, differing in the n-6:n-3 (1:1, 5:1, 10:1, 20:1), for 20 weeks. Gas chromatography-mass spectrometry was used to analyze the hepatic phospholipid arachidonic acid (AA):eicosapentaenoic acid and AA:docosahexaenoic acid. Hepatic metabolism, inflammatory signaling, macrophage polarization, gene expression of inflammatory mediators, oxidative and endoplasmic reticulum stress, and oxidative capacity were assessed as well as colonic inflammatory signaling, and gene expression of inflammatory mediators and tight-junction proteins. Although reducing the dietary n-6:n-3 lowered the hepatic phospholipid AA:eicosapentaenoic acid and AA:docosahexaenoic acid in a dose-dependent manner and mildly influenced inflammatory signaling, it did not significantly attenuate NAFLD development. Furthermore, the onset of NAFLD was not paired to colitis development or changes in tight-junction protein gene expression. In conclusion, reducing the dietary n-6:n-3 did not attenuate NAFLD progression; nor is it likely that colitis, or gut permeability, plays a role in NAFLD initiation in this model.
Keywords: Colitis; High-fat diet; Murine model; Nonalcoholic fatty-liver disease; Omega-6:omega-3; α-Linolenic acid.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
RTE, KTV, JLM, TLC, MDW, EAM do not have any conflicts of interest.
Figures

Similar articles
-
Adzuki bean ameliorates hepatic lipogenesis and proinflammatory mediator expression in mice fed a high-cholesterol and high-fat diet to induce nonalcoholic fatty liver disease.Nutr Res. 2016 Jan;36(1):90-100. doi: 10.1016/j.nutres.2015.11.002. Epub 2015 Nov 10. Nutr Res. 2016. PMID: 26773785
-
Hydroxytyrosol prevents reduction in liver activity of Δ-5 and Δ-6 desaturases, oxidative stress, and depletion in long chain polyunsaturated fatty acid content in different tissues of high-fat diet fed mice.Lipids Health Dis. 2017 Apr 11;16(1):64. doi: 10.1186/s12944-017-0450-5. Lipids Health Dis. 2017. PMID: 28395666 Free PMC article.
-
Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH).Br J Nutr. 2015 Dec 14;114(11):1745-55. doi: 10.1017/S0007114515003621. Epub 2015 Oct 9. Br J Nutr. 2015. PMID: 26450277
-
Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease.Annu Rev Nutr. 2013;33:231-48. doi: 10.1146/annurev-nutr-071812-161230. Annu Rev Nutr. 2013. PMID: 23862644 Review.
-
Potential for dietary ω-3 fatty acids to prevent nonalcoholic fatty liver disease and reduce the risk of primary liver cancer.Adv Nutr. 2015 Nov 13;6(6):694-702. doi: 10.3945/an.115.009423. Print 2015 Nov. Adv Nutr. 2015. PMID: 26567194 Free PMC article. Review.
Cited by
-
n-3 PUFAs reduce tumor load and improve survival in a NASH-tumor mouse model.Ther Adv Chronic Dis. 2019 Sep 5;10:2040622319872118. doi: 10.1177/2040622319872118. eCollection 2019. Ther Adv Chronic Dis. 2019. PMID: 31523414 Free PMC article.
-
Repeated clodronate-liposome treatment results in neutrophilia and is not effective in limiting obesity-linked metabolic impairments.Am J Physiol Endocrinol Metab. 2019 Mar 1;316(3):E358-E372. doi: 10.1152/ajpendo.00438.2018. Epub 2018 Dec 21. Am J Physiol Endocrinol Metab. 2019. PMID: 30576244 Free PMC article.
-
Loss of monocyte chemoattractant protein-1 expression delays mammary tumorigenesis and reduces localized inflammation in the C3(1)/SV40Tag triple negative breast cancer model.Cancer Biol Ther. 2017 Feb;18(2):85-93. doi: 10.1080/15384047.2016.1276135. Epub 2017 Jan 11. Cancer Biol Ther. 2017. PMID: 28075192 Free PMC article.
-
Augmenting Skeletal Muscle Estrogen Does not Prevent or Rescue Obesity-linked Metabolic Impairments in Female Mice.Endocrinology. 2022 Oct 11;163(11):bqac146. doi: 10.1210/endocr/bqac146. Endocrinology. 2022. PMID: 36039699 Free PMC article.
-
Effects of high fat diet-induced obesity on mammary tumorigenesis in the PyMT/MMTV murine model.Cancer Biol Ther. 2019;20(4):487-496. doi: 10.1080/15384047.2018.1537574. Epub 2018 Nov 2. Cancer Biol Ther. 2019. PMID: 30388923 Free PMC article.
References
-
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of nonalcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. - PubMed
-
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. - PubMed
-
- Yeh MM, Brunt EM. Pathological features of fatty liver disease. Gastroenterology. 2014;147:754–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

