Mechanism of Arctigenin-Induced Specific Cytotoxicity against Human Hepatocellular Carcinoma Cell Lines: Hep G2 and SMMC7721
- PMID: 25933104
- PMCID: PMC4416797
- DOI: 10.1371/journal.pone.0125727
Mechanism of Arctigenin-Induced Specific Cytotoxicity against Human Hepatocellular Carcinoma Cell Lines: Hep G2 and SMMC7721
Abstract
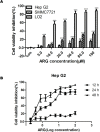
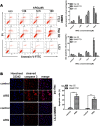
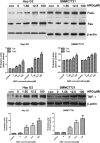
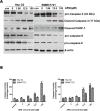
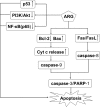
Arctigenin (ARG) has been previously reported to exert high biological activities including anti-inflammatory, antiviral and anticancer. In this study, the anti-tumor mechanism of ARG towards human hepatocellular carcinoma (HCC) was firstly investigated. We demonstrated that ARG could induce apoptosis in Hep G2 and SMMC7721 cells but not in normal hepatic cells, and its apoptotic effect on Hep G2 was stronger than that on SMMC7721. Furthermore, the following study showed that ARG treatment led to a loss in the mitochondrial out membrane potential, up-regulation of Bax, down-regulation of Bcl-2, a release of cytochrome c, caspase-9 and caspase-3 activation and a cleavage of poly (ADP-ribose) polymerase in both Hep G2 and SMMC7721 cells, suggesting ARG-induced apoptosis was associated with the mitochondria mediated pathway. Moreover, the activation of caspase-8 and the increased expression levels of Fas/FasL and TNF-α revealed that the Fas/FasL-related pathway was also involved in this process. Additionally, ARG induced apoptosis was accompanied by a deactivation of PI3K/p-Akt pathway, an accumulation of p53 protein and an inhibition of NF-κB nuclear translocation especially in Hep G2 cells, which might be the reason that Hep G2 was more sensitive than SMMC7721 cells to ARG treatment.
Conflict of interest statement
Figures


Similar articles
-
Regulation of Intrinsic and Extrinsic Apoptotic Pathways in Osteosarcoma Cells Following Oleandrin Treatment.Int J Mol Sci. 2016 Nov 23;17(11):1950. doi: 10.3390/ijms17111950. Int J Mol Sci. 2016. PMID: 27886059 Free PMC article.
-
Celastrol induces apoptosis in non-small-cell lung cancer A549 cells through activation of mitochondria- and Fas/FasL-mediated pathways.Toxicol In Vitro. 2011 Aug;25(5):1027-32. doi: 10.1016/j.tiv.2011.03.023. Epub 2011 Apr 3. Toxicol In Vitro. 2011. PMID: 21466843
-
Furazolidone induces apoptosis through activating reactive oxygen species-dependent mitochondrial signaling pathway and suppressing PI3K/Akt signaling pathway in HepG2 cells.Food Chem Toxicol. 2015 Jan;75:173-86. doi: 10.1016/j.fct.2014.11.019. Epub 2014 Nov 27. Food Chem Toxicol. 2015. PMID: 25434308
-
Apoptosis: A Target for Anticancer Therapy.Int J Mol Sci. 2018 Feb 2;19(2):448. doi: 10.3390/ijms19020448. Int J Mol Sci. 2018. PMID: 29393886 Free PMC article. Review.
-
Arctigenin, an anti-tumor agent; a cutting-edge topic and up-to-the-minute approach in cancer treatment.Eur J Pharmacol. 2021 Oct 15;909:174419. doi: 10.1016/j.ejphar.2021.174419. Epub 2021 Aug 12. Eur J Pharmacol. 2021. PMID: 34391770 Review.
Cited by
-
Arctigenin Attenuates Tumor Metastasis Through Inhibiting Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma via Suppressing GSK3β-Dependent Wnt/β-Catenin Signaling Pathway In Vivo and In Vitro.Front Pharmacol. 2019 Aug 29;10:937. doi: 10.3389/fphar.2019.00937. eCollection 2019. Front Pharmacol. 2019. PMID: 31555129 Free PMC article.
-
Investigation of the profile of phenolic compounds in the leaves and stems of Pandiaka heudelotii using gas chromatography coupled with flame ionization detector.Food Sci Nutr. 2016 Nov 23;5(3):646-652. doi: 10.1002/fsn3.443. eCollection 2017 May. Food Sci Nutr. 2016. PMID: 28572953 Free PMC article.
-
Combinatorial Effects of the Natural Products Arctigenin, Chlorogenic Acid, and Cinnamaldehyde Commit Oxidation Assassination on Breast Cancer Cells.Antioxidants (Basel). 2022 Mar 20;11(3):591. doi: 10.3390/antiox11030591. Antioxidants (Basel). 2022. PMID: 35326241 Free PMC article.
-
A Traditional Chinese Medicine Herb Mixture Qingjie Fuzheng Granules Inhibits Hepatocellular Carcinoma Cells Growth by Inducing Apoptosis.J Evid Based Integr Med. 2018 Jan-Dec;23:2515690X18789632. doi: 10.1177/2515690X18789632. J Evid Based Integr Med. 2018. PMID: 30045633 Free PMC article.
-
Arctigenin Induces an Activation Response in Porcine Alveolar Macrophage Through TLR6-NOX2-MAPKs Signaling Pathway.Front Pharmacol. 2018 May 15;9:475. doi: 10.3389/fphar.2018.00475. eCollection 2018. Front Pharmacol. 2018. PMID: 29867481 Free PMC article.
References
-
- Bruix J, Boix L, Sala M, Llovet JM. Focus on hepatocellular carcinoma. Cancer Cell. 2004; 5: 215–219. - PubMed
-
- El-Serag HB, Rudolph L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007; 132: 2557–2576. - PubMed
-
- Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nature reviews Cancer. 2006; 6: 674–687. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

