A new model of reverse cholesterol transport: enTICEing strategies to stimulate intestinal cholesterol excretion
- PMID: 25930707
- PMCID: PMC4485953
- DOI: 10.1016/j.tips.2015.04.002
A new model of reverse cholesterol transport: enTICEing strategies to stimulate intestinal cholesterol excretion
Abstract
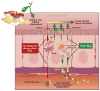
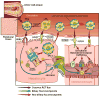
Cardiovascular disease (CVD) remains the largest cause of mortality in most developed countries. Although recent failed clinical trials and Mendelian randomization studies have called into question the high-density lipoprotein (HDL) hypothesis, it remains well accepted that stimulating the process of reverse cholesterol transport (RCT) can prevent or even regress atherosclerosis. The prevailing model for RCT is that cholesterol from the artery wall must be delivered to the liver where it is secreted into bile before leaving the body through fecal excretion. However, many studies have demonstrated that RCT can proceed through a non-biliary pathway known as transintestinal cholesterol excretion (TICE). The goal of this review is to discuss the current state of knowledge of the TICE pathway, with emphasis on points of therapeutic intervention.
Keywords: bile; cholesterol; lipoprotein; reverse cholesterol transport; transintestinal cholesterol excretion.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors report that they have no conflicts of interest.
Figures
Similar articles
-
Ezetimibe enhances macrophage reverse cholesterol transport in hamsters: contribution of hepato-biliary pathway.Biochim Biophys Acta. 2014 Sep;1841(9):1247-55. doi: 10.1016/j.bbalip.2014.05.009. Epub 2014 Jun 2. Biochim Biophys Acta. 2014. PMID: 24989153
-
Transintestinal and Biliary Cholesterol Secretion Both Contribute to Macrophage Reverse Cholesterol Transport in Rats-Brief Report.Arterioscler Thromb Vasc Biol. 2017 Apr;37(4):643-646. doi: 10.1161/ATVBAHA.116.308558. Epub 2017 Feb 23. Arterioscler Thromb Vasc Biol. 2017. PMID: 28232326
-
[Research advances of the biliary and nonbiliary pathways to reverse cholesterol transport].Sheng Li Ke Xue Jin Zhan. 2013 Apr;44(2):105-10. Sheng Li Ke Xue Jin Zhan. 2013. PMID: 23847920 Review. Chinese.
-
Reverse cholesterol transport revisited: contribution of biliary versus intestinal cholesterol excretion.Arterioscler Thromb Vasc Biol. 2011 Aug;31(8):1726-33. doi: 10.1161/ATVBAHA.108.181206. Epub 2011 May 12. Arterioscler Thromb Vasc Biol. 2011. PMID: 21571685 Review.
-
Transintestinal Cholesterol Transport Is Active in Mice and Humans and Controls Ezetimibe-Induced Fecal Neutral Sterol Excretion.Cell Metab. 2016 Dec 13;24(6):783-794. doi: 10.1016/j.cmet.2016.10.001. Epub 2016 Nov 3. Cell Metab. 2016. PMID: 27818259
Cited by
-
Intestinal basolateral lipid substrate transport is linked to chylomicron secretion and is regulated by apoC-III.J Lipid Res. 2019 Sep;60(9):1503-1515. doi: 10.1194/jlr.M092460. Epub 2019 May 31. J Lipid Res. 2019. PMID: 31152000 Free PMC article.
-
Ezetimibe Promotes Brush Border Membrane-to-Lumen Cholesterol Efflux in the Small Intestine.PLoS One. 2016 Mar 29;11(3):e0152207. doi: 10.1371/journal.pone.0152207. eCollection 2016. PLoS One. 2016. PMID: 27023132 Free PMC article.
-
Hepatic Nampt Deficiency Aggravates Dyslipidemia and Fatty Liver in High Fat Diet Fed Mice.Cells. 2023 Feb 10;12(4):568. doi: 10.3390/cells12040568. Cells. 2023. PMID: 36831235 Free PMC article.
-
Stigmasterol stimulates transintestinal cholesterol excretion independent of liver X receptor activation in the small intestine.J Nutr Biochem. 2020 Feb;76:108263. doi: 10.1016/j.jnutbio.2019.108263. Epub 2019 Nov 9. J Nutr Biochem. 2020. PMID: 31759199 Free PMC article.
-
Cholesterol Acceptors Regulate the Lipidome of Macrophage Foam Cells.Int J Mol Sci. 2019 Aug 2;20(15):3784. doi: 10.3390/ijms20153784. Int J Mol Sci. 2019. PMID: 31382484 Free PMC article.
References
-
- Go AS, et al. Executive summary: heart disease and stroke statistics – 2013 update: a report from the American Heart Association. Circulation. 2013;127:143–152. - PubMed
-
- Rader DJ, Tall AR. The not-so-simply HDL story: Is it time to revise the HDL cholesterol hypothesis? Nat Med. 2012;18:1344–1346. - PubMed
-
- Boden WE, et al. AIM-HIGH investigators. (2011) Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 365:2255–2267. - PubMed
-
- Schwartz GG, et al. Effect of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

