Protective Effect of Administered Rolipram against Radiation-Induced Testicular Injury in Mice
- PMID: 25927059
- PMCID: PMC4412004
- DOI: 10.5534/wjmh.2015.33.1.20
Protective Effect of Administered Rolipram against Radiation-Induced Testicular Injury in Mice
Abstract
Purpose: Pelvic irradiation for the treatment of cancer can affect normal cells, such as the rapidly proliferating spermatogenic cells of the testis, leading to infertility, a common post-irradiation problem. The present study investigated the radioprotective effect of rolipram, a specific phosphodiesterase type-IV inhibitor known to increase the expression and phosphorylation of the cyclic adenosine monophosphate response element-binding protein (CREB), a key factor for spermatogenesis, with the testicular system against pelvic irradiation.
Materials and methods: Male C57BL/6 mice were treated with pelvic irradiation (2 Gy) and rolipram, alone or in combination, and were sacrificed at 12 hours and 35 days after irradiation.
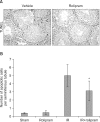
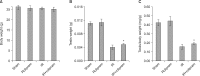
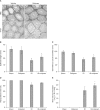
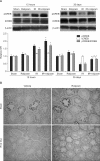
Results: Rolipram protected germ cells from radiation-induced apoptosis at 12 hours after irradiation and significantly increased testis weight compared with irradiation controls at 35 days. Rolipram also ameliorated radiation-induced testicular morphological changes, such as changes in seminiferous tubular diameter and epithelial height. Additionally, seminiferous tubule repopulation and stem cell survival indices were higher in the rolipram-treated group than in the radiation group. Moreover, rolipram treatment counteracted the radiation-mediated decrease in the sperm count and mobility in the epididymis.
Conclusions: These protective effects of rolipram treatment prior to irradiation may be mediated by the increase in pCREB levels at 12 hours post-irradiation and the attenuated decrease in pCREB levels in the testis at 35 days post-irradiation in the rolipram-treated group. These findings suggest that activation of CREB signaling by rolipram treatment ameliorates the detrimental effects of acute irradiation on testicular dysfunction and the related male reproductive functions in mice.
Keywords: Cyclic AMP response element-binding protein; Radiation; Rolipram; Testis.
Conflict of interest statement
Figures

Similar articles
-
Genistein mitigates radiation-induced testicular injury.Phytother Res. 2012 Aug;26(8):1119-25. doi: 10.1002/ptr.3689. Epub 2011 Dec 9. Phytother Res. 2012. PMID: 22162311
-
Protection of spermatogenesis against gamma ray-induced damage by granulocyte colony-stimulating factor in mice.Andrologia. 2011 Apr;43(2):87-93. doi: 10.1111/j.1439-0272.2009.01023.x. Epub 2010 Dec 29. Andrologia. 2011. PMID: 21382061
-
Carnosine mitigates apoptosis and protects testicular seminiferous tubules from gamma-radiation-induced injury in mice.Andrologia. 2014;46(9):1041-6. doi: 10.1111/and.12193. Epub 2013 Nov 12. Andrologia. 2014. PMID: 24215656
-
Irradiation affects germ and somatic cells in prepubertal monkey testis xenografts.Mol Hum Reprod. 2017 Mar 1;23(3):141-154. doi: 10.1093/molehr/gax003. Mol Hum Reprod. 2017. PMID: 28130393
-
Spermatogenesis by Sisyphus: proliferating stem germ cells fail to repopulate the testis after 'irreversible' injury.Adv Exp Med Biol. 2001;500:421-8. doi: 10.1007/978-1-4615-0667-6_64. Adv Exp Med Biol. 2001. PMID: 11764975 Review.
Cited by
-
Reduced Glycolysis Contributed to Inhibition of Testis Spermatogenesis in Rats After Chronic Methamphetamine Exposure.Med Sci Monit. 2019 Jul 23;25:5453-5464. doi: 10.12659/MSM.917491. Med Sci Monit. 2019. PMID: 31332157 Free PMC article.
-
Polydatin Alleviates Radiation-Induced Testes Injury by Scavenging ROS and Inhibiting Apoptosis Pathways.Med Sci Monit. 2018 Dec 12;24:8993-9000. doi: 10.12659/MSM.913725. Med Sci Monit. 2018. PMID: 30539937 Free PMC article.
-
Targeting CREB in Cancer Therapy: A Key Candidate or One of Many? An Update.Cancers (Basel). 2020 Oct 28;12(11):3166. doi: 10.3390/cancers12113166. Cancers (Basel). 2020. PMID: 33126560 Free PMC article. Review.
-
Differential Effects of Low and High Radiation Dose Rates on Mouse Spermatogenesis.Int J Mol Sci. 2021 Nov 27;22(23):12834. doi: 10.3390/ijms222312834. Int J Mol Sci. 2021. PMID: 34884637 Free PMC article.
-
Protective effects of an aqueous extract of Protaetia brevitarsis seulensis larvae against radiation-induced testicular injury in mice.Food Sci Nutr. 2022 Sep 20;10(11):3969-3978. doi: 10.1002/fsn3.2992. eCollection 2022 Nov. Food Sci Nutr. 2022. PMID: 36348800 Free PMC article.
References
-
- Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr. 2005;(34):12–17. - PubMed
-
- Budgell GJ, Cowan RA, Hounsell AR. Prediction of scattered dose to the testes in abdominopelvic radiotherapy. Clin Oncol (R Coll Radiol) 2001;13:120–125. - PubMed
-
- Kovacs GT, Stern K. Reproductive aspects of cancer treatment: an update. Med J Aust. 1999;170:495–497. - PubMed
-
- Costabile RA. The effects of cancer and cancer therapy on male reproductive function. J Urol. 1993;149:1327–1330. - PubMed
-
- Montminy M. Transcriptional activation. Something new to hang your HAT on. Nature. 1997;387:654–655. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

