Regulation of PGC-1α Isoform Expression in Skeletal Muscles
- PMID: 25927001
- PMCID: PMC4410395
Regulation of PGC-1α Isoform Expression in Skeletal Muscles
Abstract
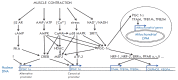
The coactivator PGC-1α is the key regulator of mitochondrial biogenesis in skeletal muscle. Skeletal muscle expresses several PGC-1α isoforms. This review covers the functional role of PGC-1α isoforms and the regulation of their exercise-associated expression in skeletal muscle. The patterns of PGC-1α mRNA expression may markedly differ at rest and after muscle activity. Different signaling pathways are activated by different physiological stimuli, which regulate the expression of the PGC-1α gene from the canonical and alternative promoters: expression from a canonical (proximal) promoter is regulated by activation of the AMPK; expression from an alternative promoter, via a β2-adrenergic receptor. All transcripts from both promoters are subject to alternative splicing. As a result, truncated isoforms that possess different properties are translated: truncated isoforms are more stable and predominantly activate angiogenesis, whereas full-length isoforms manly regulate mitochondrial biogenesis. The existence of several isoforms partially explains the broad-spectrum function of this protein and allows the organism to adapt to different physiological stimuli. Regulation of the PGC-1α gene expression by different signaling pathways provides ample opportunity for pharmacological influence on the expression of this gene. Those opportunities might be important for the treatment and prevention of various diseases, such as metabolic syndrome and diabetes mellitus. Elucidation of the regulatory mechanisms of the PGC-1α gene expression and their functional role may provide an opportunity to control the expression of different isoforms through exercise and/or pharmacological intervention.
Keywords: PGC-1α; alternative promoter; alternative splicing; gene expression; skeletal muscle.
Figures

Similar articles
-
Isoform-specific increases in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to beta2-adrenergic receptor activation and exercise.Endocrinology. 2008 Sep;149(9):4527-33. doi: 10.1210/en.2008-0466. Epub 2008 May 29. Endocrinology. 2008. PMID: 18511502
-
Epigenetic Modifications of the PGC-1α Promoter during Exercise Induced Expression in Mice.PLoS One. 2015 Jun 8;10(6):e0129647. doi: 10.1371/journal.pone.0129647. eCollection 2015. PLoS One. 2015. PMID: 26053857 Free PMC article.
-
AMPK does not play a requisite role in regulation of PPARGC1A gene expression via the alternative promoter in endurance-trained human skeletal muscle.Exp Physiol. 2017 Mar 1;102(3):366-375. doi: 10.1113/EP086074. Epub 2017 Feb 9. Exp Physiol. 2017. PMID: 28074493
-
Adaptation of Skeletal Muscles to Contractile Activity of Varying Duration and Intensity: The Role of PGC-1α.Biochemistry (Mosc). 2018 Jun;83(6):613-628. doi: 10.1134/S0006297918060019. Biochemistry (Mosc). 2018. PMID: 30195320 Review.
-
The hitchhiker's guide to PGC-1α isoform structure and biological functions.Diabetologia. 2015 Sep;58(9):1969-77. doi: 10.1007/s00125-015-3671-z. Epub 2015 Jun 25. Diabetologia. 2015. PMID: 26109214 Review.
Cited by
-
PGC-1α activation: a therapeutic target for type 2 diabetes?Eat Weight Disord. 2019 Jun;24(3):385-395. doi: 10.1007/s40519-018-0622-y. Epub 2018 Nov 29. Eat Weight Disord. 2019. PMID: 30498989 Review.
-
Epigenetics of Mitochondria-Associated Genes in Striated Muscle.Epigenomes. 2021 Dec 22;6(1):1. doi: 10.3390/epigenomes6010001. Epigenomes. 2021. PMID: 35076500 Free PMC article.
-
Quercetin and Quercetin-Rich Red Onion Extract Alter Pgc-1α Promoter Methylation and Splice Variant Expression.PPAR Res. 2017;2017:3235693. doi: 10.1155/2017/3235693. Epub 2017 Jan 16. PPAR Res. 2017. PMID: 28191013 Free PMC article.
-
Potential regulatory role of PGC-1α within the skeletal muscle during metabolic adaptations in response to high-fat diet feeding in animal models.Pflugers Arch. 2024 Mar;476(3):283-293. doi: 10.1007/s00424-023-02890-0. Epub 2023 Dec 4. Pflugers Arch. 2024. PMID: 38044359 Free PMC article. Review.
-
DNA methylation of exercise-responsive genes differs between trained and untrained men.BMC Biol. 2024 Jul 4;22(1):147. doi: 10.1186/s12915-024-01938-6. BMC Biol. 2024. PMID: 38965555 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
