The therapeutic potential of orphan GPCRs, GPR35 and GPR55
- PMID: 25926795
- PMCID: PMC4397721
- DOI: 10.3389/fphar.2015.00069
The therapeutic potential of orphan GPCRs, GPR35 and GPR55
Abstract
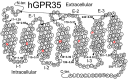
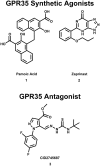
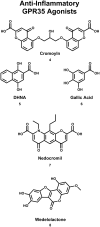
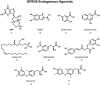
The G protein-coupled receptor (GPCR) superfamily of integral proteins is the largest family of signal transducers, comprised of ∼1000 members. Considering their prevalence and functional importance, it's not surprising that ∼60% of drugs target GPCRs. Regardless, there exists a subset of the GPCR superfamily that is largely uncharacterized and poorly understood; specifically, more than 140 GPCRs have unknown endogenous ligands-the so-called orphan GPCRs. Orphan GPCRs offer tremendous promise, as they may provide novel therapeutic targets that may be more selective than currently known receptors, resulting in the potential reduction in side effects. In addition, they may provide access to signal transduction pathways currently unknown, allowing for new strategies in drug design. Regardless, orphan GPCRs are an important area of inquiry, as they represent a large gap in our understanding of signal transduction at the cellular level. Here, we focus on the therapeutic potential of two recently deorphanized GPCRs: GPR35/CXCR8 and GPR55. First, GPR35/CXCR8 has been observed in numerous tissues/organ systems, including the gastrointestinal tract, liver, immune system, central nervous system, and cardiovascular system. Not surprisingly, GPR35/CXCR8 has been implicated in numerous pathologies involving these tissues/systems. While several endogenous ligands have been identified, GPR35/CXCR8 has recently been observed to bind the chemokine CXCL17. Second, GPR55 has been observed to be expressed in the central nervous system, adrenal glands, gastrointestinal tract, lung, liver, uterus, bladder, kidney, and bone, as well as, other tissues/organ systems. Likewise, it is not surprising that GPR55 has been implicated in pathologies involving these tissues/systems. GPR55 was initially deorphanized as a cannabinoid receptor and this receptor does bind many cannabinoid compounds. However, the GPR55 endogenous ligand has been found to be a non-cannabinoid, lysophophatidylinositol (LPI) and subsequent high throughput assays have identified other GPR55 ligands that are not cannabinoids and do not bind to either the cannabinoid CB1 and CB2 receptors. Here, we review reports that suggest that GPR35/CXCR8 and GPR55 may be promising therapeutic targets, with diverse physiological roles.
Keywords: 2-oleoyl LPA; CXCR8; GPR35; GPR55; LPI; kynurenic acid.
Figures



Similar articles
-
GPR55 and GPR35 and their relationship to cannabinoid and lysophospholipid receptors.Life Sci. 2013 Mar 19;92(8-9):453-7. doi: 10.1016/j.lfs.2012.06.039. Epub 2012 Jul 20. Life Sci. 2013. PMID: 22820167 Review.
-
Screening for Selective Ligands for GPR55 - Agonists.2010 Sep 29 [updated 2011 May 26]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. 2010 Sep 29 [updated 2011 May 26]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 22091480 Free Books & Documents. Review.
-
The role and clinical significance of the CXCL17-CXCR8 (GPR35) axis in breast cancer.Biochem Biophys Res Commun. 2017 Nov 25;493(3):1159-1167. doi: 10.1016/j.bbrc.2017.09.113. Epub 2017 Sep 21. Biochem Biophys Res Commun. 2017. PMID: 28943434
-
Screening for Selective Ligands for GPR55 - Antagonists.2010 Oct 30 [updated 2011 May 26]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. 2010 Oct 30 [updated 2011 May 26]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 22091481 Free Books & Documents. Review.
-
Identification of the GPR55 antagonist binding site using a novel set of high-potency GPR55 selective ligands.Biochemistry. 2013 Dec 31;52(52):9456-69. doi: 10.1021/bi4008885. Epub 2013 Dec 17. Biochemistry. 2013. PMID: 24274581 Free PMC article.
Cited by
-
Endocannabinoid-Binding Receptors as Drug Targets.Methods Mol Biol. 2023;2576:67-94. doi: 10.1007/978-1-0716-2728-0_6. Methods Mol Biol. 2023. PMID: 36152178
-
Cannabinoids: Potential for Modulation and Enhancement When Combined with Vitamin B12 in Case of Neurodegenerative Disorders.Pharmaceuticals (Basel). 2024 Jun 20;17(6):813. doi: 10.3390/ph17060813. Pharmaceuticals (Basel). 2024. PMID: 38931480 Free PMC article. Review.
-
The Role of Cannabidiol in Liver Disease: A Systemic Review.Int J Mol Sci. 2024 Feb 17;25(4):2370. doi: 10.3390/ijms25042370. Int J Mol Sci. 2024. PMID: 38397045 Free PMC article. Review.
-
Kynurenines and the Endocannabinoid System in Schizophrenia: Common Points and Potential Interactions.Molecules. 2019 Oct 15;24(20):3709. doi: 10.3390/molecules24203709. Molecules. 2019. PMID: 31619006 Free PMC article. Review.
-
Discovery of Kynurenines Containing Oligopeptides as Potent Opioid Receptor Agonists.Biomolecules. 2020 Feb 12;10(2):284. doi: 10.3390/biom10020284. Biomolecules. 2020. PMID: 32059524 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

