Impaired Th17 polarization of phenotypically naive CD4(+) T-cells during chronic HIV-1 infection and potential restoration with early ART
- PMID: 25924895
- PMCID: PMC4438463
- DOI: 10.1186/s12977-015-0164-6
Impaired Th17 polarization of phenotypically naive CD4(+) T-cells during chronic HIV-1 infection and potential restoration with early ART
Abstract
Background: Depletion of mucosal Th17 cells during HIV/SIV infections is a major cause for microbial translocation, chronic immune activation, and disease progression. Mechanisms contributing to Th17 deficit are not fully elucidated. Here we investigated alterations in the Th17 polarization potential of naive-like CD4(+) T-cells, depletion of Th17-commited subsets during HIV pathogenesis, and Th17 restoration in response to antiretroviral therapy (ART).
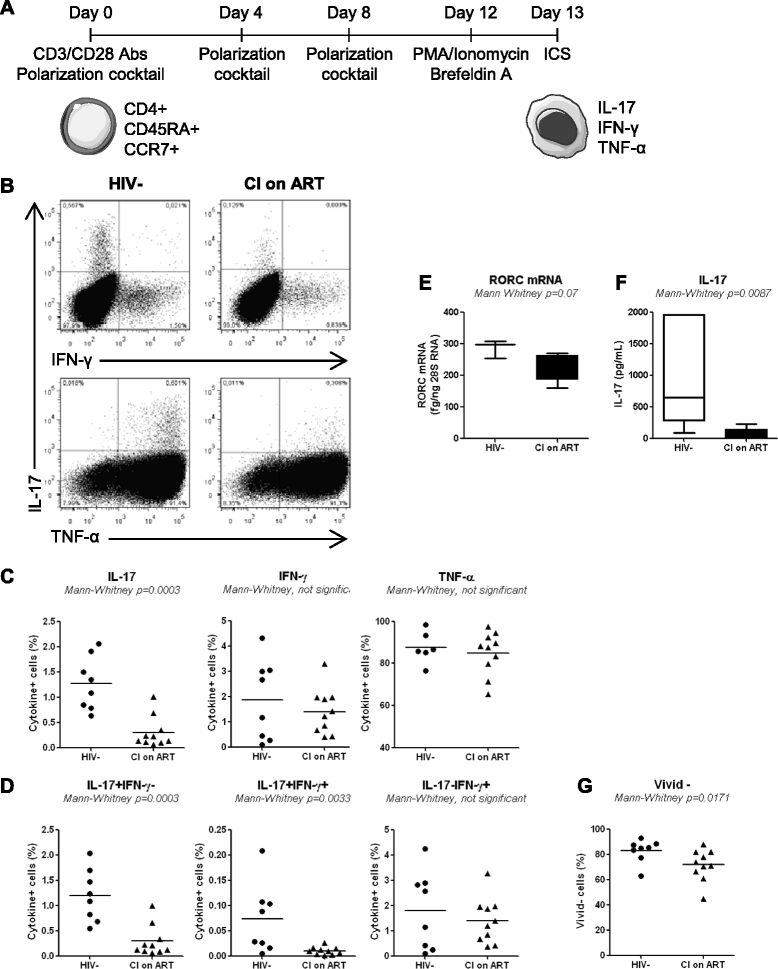
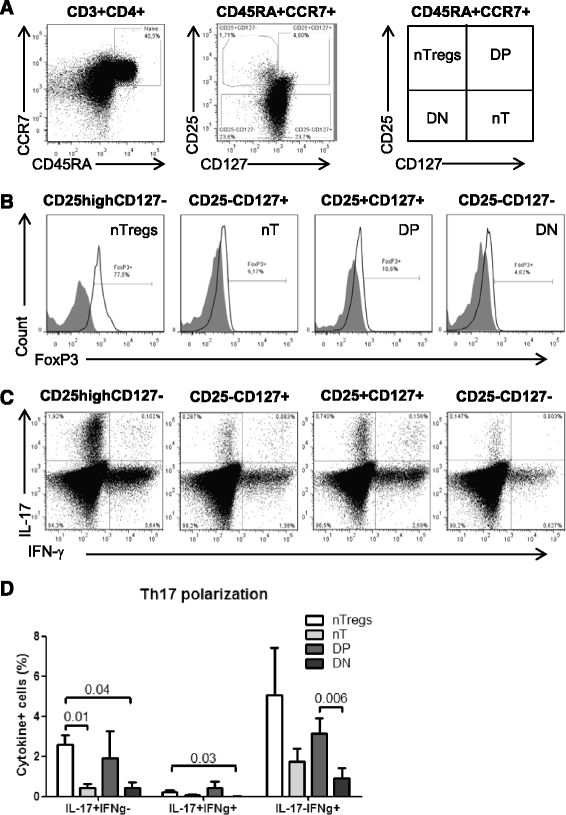
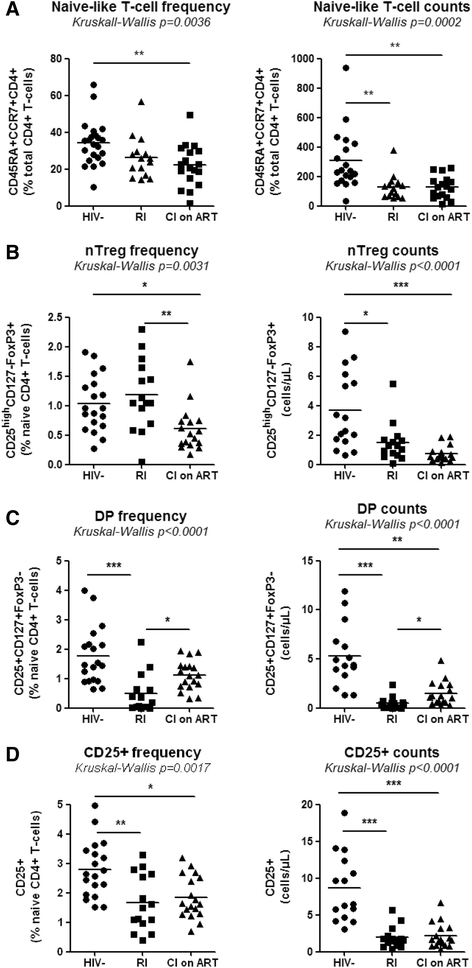
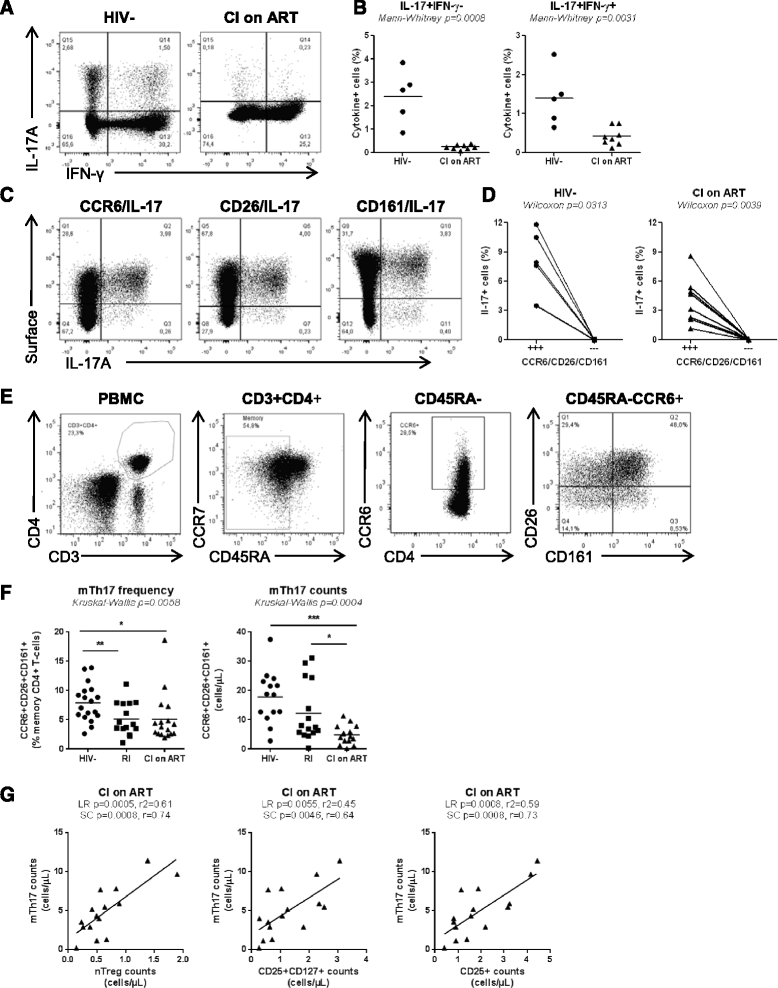
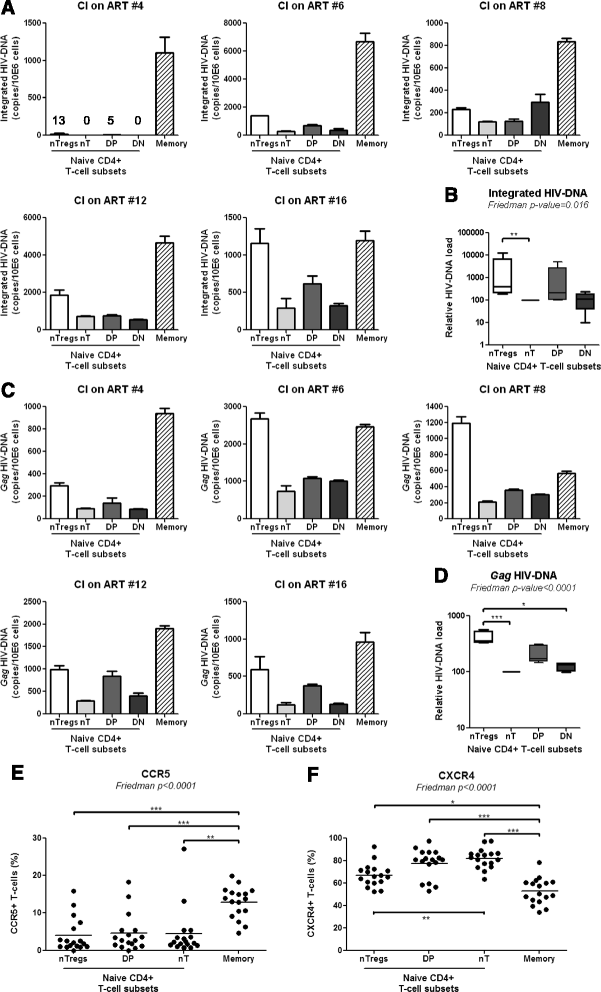
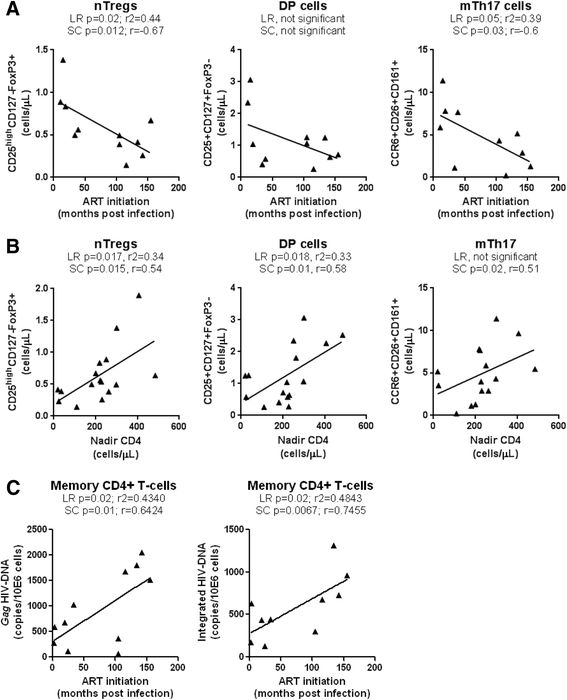
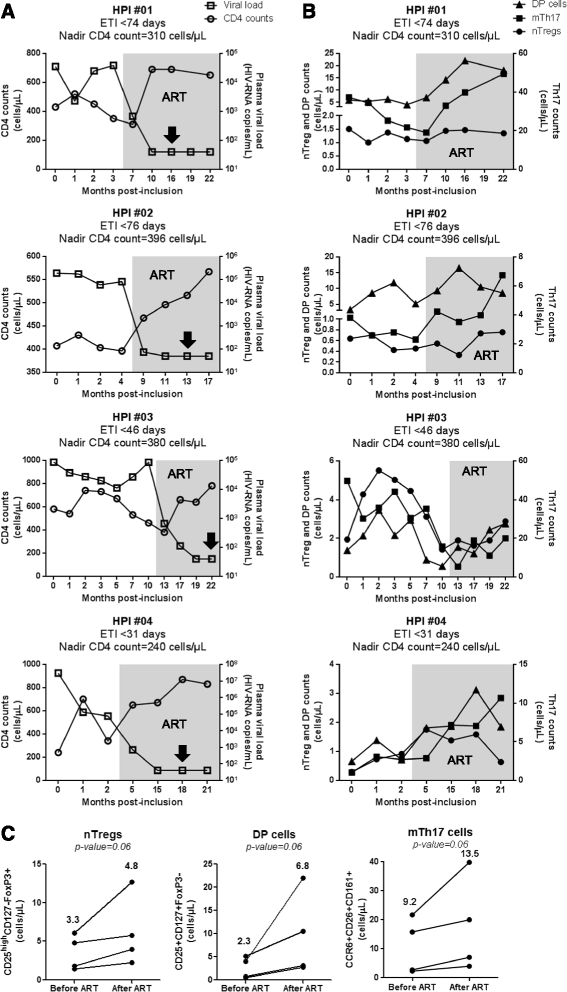
Results: Peripheral blood CD4(+) T-cells expressing a naive-like phenotype (CD45RA(+)CCR7(+)) from chronically HIV-infected subjects receiving ART (CI on ART; median CD4 counts 592 cells/μl; viral load: <50 HIV-RNA copies/ml; time since infection: 156 months) compared to uninfected controls (HIV-) were impaired in their survival and Th17 polarization potential in vitro. In HIV- controls, IL-17A-producing cells mainly originated from naive-like T-cells with a regulatory phenotype (nTregs: CD25(high)CD127(-)FoxP3(+)) and from CD25(+)CD127(+)FoxP3(-) cells (DP, double positive). Th17-polarized conventional naive CD4(+) T-cells (nT: CD25(-)CD127(+)FoxP3(-)) also produced IL17A, but at lower frequency compared to nTregs and DP. In CI on ART subjects, the frequency/counts of nTreg and DP were significantly diminished compared to HIV- controls, and this paucity was further associated with decreased proportions of memory T-cells producing IL-17A and expressing Th17 markers (CCR6(+)CD26(+)CD161(+), mTh17). nTregs and DP compared to nT cells harbored superior levels of integrated/non-integrated HIV-DNA in CI on ART subjects, suggesting that permissiveness to integrative/abortive infection contributes to impaired survival and Th17 polarization of lineage-committed cells. A cross-sectional study in CI on ART subjects revealed that nTregs, DP and mTh17 counts were negatively correlated with the time post-infection ART was initiated and positively correlated with nadir CD4 counts. Finally, a longitudinal analysis in a HIV primary infection cohort demonstrated a tendency for increased nTreg, DP, and mTh17 counts with ART initiation during the first year of infection.
Conclusions: These results support a model in which the paucity of phenotypically naive nTregs and DP cells, caused by integrative/abortive HIV infection and/or other mechanisms, contributes to Th17 deficiency in HIV-infected subjects. Early ART initiation, treatment intensification with integrase inhibitors, and/or other alternative interventions aimed at preserving/restoring the pool of cells prone to acquire Th17 functions may significantly improve mucosal immunity in HIV-infected subjects.
Figures
Similar articles
-
Naive and Memory CD4⁺ T Cells Are Differentially Affected in Indonesian HIV Patients Responding to ART.Viral Immunol. 2016 Apr;29(3):176-83. doi: 10.1089/vim.2015.0108. Viral Immunol. 2016. PMID: 27035638
-
Interleukin-7 signalling defects in naive CD4+ T cells of HIV patients with CD4+ T-cell deficiency on antiretroviral therapy are associated with T-cell activation and senescence.AIDS. 2014 Mar 27;28(6):821-30. doi: 10.1097/QAD.0000000000000213. AIDS. 2014. PMID: 24499954
-
New insights into the heterogeneity of Th17 subsets contributing to HIV-1 persistence during antiretroviral therapy.Retrovirology. 2016 Aug 24;13(1):59. doi: 10.1186/s12977-016-0293-6. Retrovirology. 2016. PMID: 27553844 Free PMC article.
-
Immune activation during acute HIV infection and the impact of early antiretroviral therapy.Curr Opin HIV AIDS. 2016 Mar;11(2):163-72. doi: 10.1097/COH.0000000000000228. Curr Opin HIV AIDS. 2016. PMID: 26599167 Review.
-
Blocking Formation of the Stable HIV Reservoir: A New Perspective for HIV-1 Cure.Front Immunol. 2019 Aug 22;10:1966. doi: 10.3389/fimmu.2019.01966. eCollection 2019. Front Immunol. 2019. PMID: 31507594 Free PMC article. Review.
Cited by
-
Differentiating Immune Cell Targets in Gut-Associated Lymphoid Tissue for HIV Cure.AIDS Res Hum Retroviruses. 2017 Nov;33(S1):S40-S58. doi: 10.1089/AID.2017.0153. AIDS Res Hum Retroviruses. 2017. PMID: 28882067 Free PMC article. Review.
-
Benefit delayed immunosenescence by regulating CD4+T cells: A promising therapeutic target for aging-related diseases.Aging Cell. 2024 Oct;23(10):e14317. doi: 10.1111/acel.14317. Epub 2024 Aug 18. Aging Cell. 2024. PMID: 39155409 Free PMC article. Review.
-
Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection.AIDS Res Ther. 2016 Apr 11;13:19. doi: 10.1186/s12981-016-0103-1. eCollection 2016. AIDS Res Ther. 2016. PMID: 27073405 Free PMC article. Review.
-
Preservation of Gastrointestinal Mucosal Barrier Function and Microbiome in Patients With Controlled HIV Infection.Front Immunol. 2021 May 31;12:688886. doi: 10.3389/fimmu.2021.688886. eCollection 2021. Front Immunol. 2021. PMID: 34135912 Free PMC article.
-
Alterations in Th17 Cells and Non-Classical Monocytes as a Signature of Subclinical Coronary Artery Atherosclerosis during ART-Treated HIV-1 Infection.Cells. 2024 Jan 15;13(2):157. doi: 10.3390/cells13020157. Cells. 2024. PMID: 38247848 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

