Hydration water mobility is enhanced around tau amyloid fibers
- PMID: 25918405
- PMCID: PMC4443308
- DOI: 10.1073/pnas.1422824112
Hydration water mobility is enhanced around tau amyloid fibers
Abstract
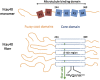
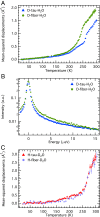
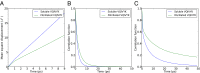
The paired helical filaments (PHF) formed by the intrinsically disordered human protein tau are one of the pathological hallmarks of Alzheimer disease. PHF are fibers of amyloid nature that are composed of a rigid core and an unstructured fuzzy coat. The mechanisms of fiber formation, in particular the role that hydration water might play, remain poorly understood. We combined protein deuteration, neutron scattering, and all-atom molecular dynamics simulations to study the dynamics of hydration water at the surface of fibers formed by the full-length human protein htau40. In comparison with monomeric tau, hydration water on the surface of tau fibers is more mobile, as evidenced by an increased fraction of translationally diffusing water molecules, a higher diffusion coefficient, and increased mean-squared displacements in neutron scattering experiments. Fibers formed by the hexapeptide (306)VQIVYK(311) were taken as a model for the tau fiber core and studied by molecular dynamics simulations, revealing that hydration water dynamics around the core domain is significantly reduced after fiber formation. Thus, an increase in water dynamics around the fuzzy coat is proposed to be at the origin of the experimentally observed increase in hydration water dynamics around the entire tau fiber. The observed increase in hydration water dynamics is suggested to promote fiber formation through entropic effects. Detection of the enhanced hydration water mobility around tau fibers is conjectured to potentially contribute to the early diagnosis of Alzheimer patients by diffusion MRI.
Keywords: amyloid fibers; hydration water; intrinsically disordered proteins; neutron scattering; tau protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Translational diffusion of hydration water correlates with functional motions in folded and intrinsically disordered proteins.Nat Commun. 2015 Mar 16;6:6490. doi: 10.1038/ncomms7490. Nat Commun. 2015. PMID: 25774711 Free PMC article.
-
Diffusion of Hydration Water around Intrinsically Disordered Proteins.J Phys Chem B. 2015 Oct 22;119(42):13262-70. doi: 10.1021/acs.jpcb.5b07248. Epub 2015 Oct 8. J Phys Chem B. 2015. PMID: 26418258
-
Molecular Dynamics Simulations of a Powder Model of the Intrinsically Disordered Protein Tau.J Phys Chem B. 2015 Oct 1;119(39):12580-9. doi: 10.1021/acs.jpcb.5b05849. Epub 2015 Sep 18. J Phys Chem B. 2015. PMID: 26351734
-
Role of water in protein folding, oligomerization, amyloidosis and miniprotein.J Pept Sci. 2014 Oct;20(10):747-59. doi: 10.1002/psc.2671. Epub 2014 Aug 6. J Pept Sci. 2014. PMID: 25098401 Review.
-
Computational studies of protein aggregation: methods and applications.Annu Rev Phys Chem. 2015 Apr;66:643-66. doi: 10.1146/annurev-physchem-040513-103738. Epub 2015 Feb 2. Annu Rev Phys Chem. 2015. PMID: 25648485 Review.
Cited by
-
Amyloid-Like Aggregation in Diseases and Biomaterials: Osmosis of Structural Information.Front Bioeng Biotechnol. 2021 Mar 3;9:641372. doi: 10.3389/fbioe.2021.641372. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33748087 Free PMC article. Review.
-
Water Determines the Structure and Dynamics of Proteins.Chem Rev. 2016 Jul 13;116(13):7673-97. doi: 10.1021/acs.chemrev.5b00664. Epub 2016 May 17. Chem Rev. 2016. PMID: 27186992 Free PMC article. Review.
-
Water Distribution, Dynamics, and Interactions with Alzheimer's β-Amyloid Fibrils Investigated by Solid-State NMR.J Am Chem Soc. 2017 May 3;139(17):6242-6252. doi: 10.1021/jacs.7b02089. Epub 2017 Apr 21. J Am Chem Soc. 2017. PMID: 28406028 Free PMC article.
-
Amyloid Oligomers: A Joint Experimental/Computational Perspective on Alzheimer's Disease, Parkinson's Disease, Type II Diabetes, and Amyotrophic Lateral Sclerosis.Chem Rev. 2021 Feb 24;121(4):2545-2647. doi: 10.1021/acs.chemrev.0c01122. Epub 2021 Feb 5. Chem Rev. 2021. PMID: 33543942 Free PMC article. Review.
-
Diffusion Tensor Imaging Detects Acute Pathology-Specific Changes in the P301L Tauopathy Mouse Model Following Traumatic Brain Injury.Front Neurosci. 2021 Feb 24;15:611451. doi: 10.3389/fnins.2021.611451. eCollection 2021. Front Neurosci. 2021. PMID: 33716645 Free PMC article.
References
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75(1):333–366. - PubMed
-
- Uversky VN, Oldfield CJ, Dunker AK. Intrinsically disordered proteins in human diseases: Introducing the D2 concept. Annu Rev Biophys. 2008;37(1):215–246. - PubMed
-
- Brion JP, Couck AM, Passareiro E, Flament-Durand J. Neurofibrillary tangles of Alzheimer’s disease: An immunohistochemical study. J Submicrosc Cytol. 1985;17(1):89–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

