ICOS and Bcl6-dependent pathways maintain a CD4 T cell population with memory-like properties during tuberculosis
- PMID: 25918344
- PMCID: PMC4419347
- DOI: 10.1084/jem.20141518
ICOS and Bcl6-dependent pathways maintain a CD4 T cell population with memory-like properties during tuberculosis
Abstract
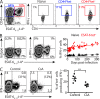
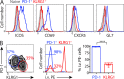
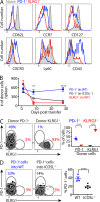
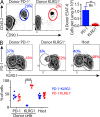
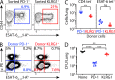
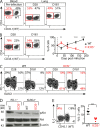
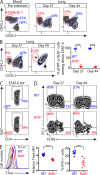
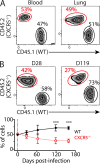
Immune control of persistent infection with Mycobacterium tuberculosis (Mtb) requires a sustained pathogen-specific CD4 T cell response; however, the molecular pathways governing the generation and maintenance of Mtb protective CD4 T cells are poorly understood. Using MHCII tetramers, we show that Mtb-specific CD4 T cells are subject to ongoing antigenic stimulation. Despite this chronic stimulation, a subset of PD-1(+) cells is maintained within the lung parenchyma during tuberculosis (TB). When transferred into uninfected animals, these cells persist, mount a robust recall response, and provide superior protection to Mtb rechallenge when compared to terminally differentiated Th1 cells that reside preferentially in the lung-associated vasculature. The PD-1(+) cells share features with memory CD4 T cells in that their generation and maintenance requires intrinsic Bcl6 and intrinsic ICOS expression. Thus, the molecular pathways required to maintain Mtb-specific CD4 T cells during ongoing infection are similar to those that maintain memory CD4 T cells in scenarios of antigen deprivation. These results suggest that vaccination strategies targeting the ICOS and Bcl6 pathways in CD4 T cells may provide new avenues to prevent TB.
© 2015 Moguche et al.
Figures


Similar articles
-
The α4β1 integrin in localization of Mycobacterium tuberculosis-specific T helper type 1 cells to the human lung.Am J Respir Cell Mol Biol. 2011 Jul;45(1):24-30. doi: 10.1165/rcmb.2010-0241OC. Epub 2010 Aug 19. Am J Respir Cell Mol Biol. 2011. PMID: 20724551
-
Suppressed induction of mycobacterial antigen-specific Th1-type CD4+ T cells in the lung after pulmonary mycobacterial infection.Int Immunol. 2010 Apr;22(4):307-18. doi: 10.1093/intimm/dxq010. Epub 2010 Feb 18. Int Immunol. 2010. PMID: 20167585
-
Protective immunity afforded by attenuated, PhoP-deficient Mycobacterium tuberculosis is associated with sustained generation of CD4+ T-cell memory.Eur J Immunol. 2012 Feb;42(2):385-92. doi: 10.1002/eji.201141903. Epub 2011 Dec 27. Eur J Immunol. 2012. PMID: 22105536
-
Immunological protection against mycobacterium tuberculosis infection.Crit Rev Immunol. 2006;26(6):515-26. doi: 10.1615/critrevimmunol.v26.i6.40. Crit Rev Immunol. 2006. PMID: 17341192 Review.
-
Pro- and anti-inflammatory cytokines in tuberculosis: a two-edged sword in TB pathogenesis.Semin Immunol. 2014 Dec;26(6):543-51. doi: 10.1016/j.smim.2014.09.011. Epub 2014 Nov 8. Semin Immunol. 2014. PMID: 25453229 Review.
Cited by
-
BCL6-dependent TCF-1+ progenitor cells maintain effector and helper CD4+ T cell responses to persistent antigen.Immunity. 2022 Jul 12;55(7):1200-1215.e6. doi: 10.1016/j.immuni.2022.05.003. Epub 2022 May 27. Immunity. 2022. PMID: 35637103 Free PMC article.
-
Limited Antimycobacterial Efficacy of Epitope Peptide Administration Despite Enhanced Antigen-Specific CD4 T-Cell Activation.J Infect Dis. 2018 Oct 5;218(10):1653-1662. doi: 10.1093/infdis/jiy142. J Infect Dis. 2018. PMID: 29548008 Free PMC article.
-
Targeting Mycobacterium tuberculosis Antigens to Dendritic Cells via the DC-Specific-ICAM3-Grabbing-Nonintegrin Receptor Induces Strong T-Helper 1 Immune Responses.Front Immunol. 2018 Mar 9;9:471. doi: 10.3389/fimmu.2018.00471. eCollection 2018. Front Immunol. 2018. PMID: 29662482 Free PMC article.
-
Characterization of the Antigen-Specific CD4(+) T Cell Response Induced by Prime-Boost Strategies with CAF01 and CpG Adjuvants Administered by the Intranasal and Subcutaneous Routes.Front Immunol. 2015 Aug 28;6:430. doi: 10.3389/fimmu.2015.00430. eCollection 2015. Front Immunol. 2015. PMID: 26379666 Free PMC article.
-
Peripheral and lung resident memory T cell responses against SARS-CoV-2.Nat Commun. 2021 May 21;12(1):3010. doi: 10.1038/s41467-021-23333-3. Nat Commun. 2021. PMID: 34021148 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

