Expression of GM-CSF in T Cells Is Increased in Multiple Sclerosis and Suppressed by IFN-β Therapy
- PMID: 25917097
- PMCID: PMC4433790
- DOI: 10.4049/jimmunol.1403243
Expression of GM-CSF in T Cells Is Increased in Multiple Sclerosis and Suppressed by IFN-β Therapy
Abstract
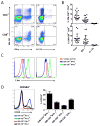
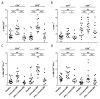
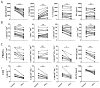
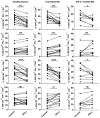
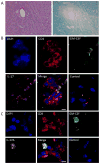
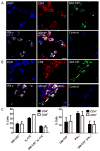
Multiple sclerosis (MS) is an autoimmune disease of the CNS. Studies in animal models of MS have shown that GM-CSF produced by T cells is necessary for the development of autoimmune CNS inflammation. This suggests that GM-CSF may have a pathogenic role in MS as well, and a clinical trial testing its blockade is ongoing. However, there have been few reports on GM-CSF production by T cells in MS. The objective of this study was to characterize GM-CSF production by T cells of MS patients and to determine the effect of IFN-β therapy on its production. GM-CSF production by peripheral blood (PB) T cells and the effects of IFN-β were characterized in samples of untreated and IFN-β-treated MS patients versus healthy subjects. GM-CSF production by T cells in MS brain lesions was analyzed by immunofluorescence. Untreated MS patients had significantly greater numbers of GM-CSF(+)CD4(+) and CD8(+) T cells in PB compared with healthy controls and IFN-β-treated MS patients. IFN-β significantly suppressed GM-CSF production by T cells in vitro. A number of CD4(+) and CD8(+) T cells in MS brain lesions expressed GM-CSF. Elevated GM-CSF production by PB T cells in MS is indicative of aberrant hyperactivation of the immune system. Given its essential role in animal models, abundant GM-CSF production at the sites of CNS inflammation suggests that GM-CSF contributes to MS pathogenesis. Our findings also reveal a potential mechanism of IFN-β therapy, namely suppression of GM-CSF production.
Copyright © 2015 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures

Similar articles
-
Role of Granulocyte-Macrophage Colony-Stimulating Factor Production by T Cells during Mycobacterium tuberculosis Infection.mBio. 2017 Oct 24;8(5):e01514-17. doi: 10.1128/mBio.01514-17. mBio. 2017. PMID: 29066547 Free PMC article.
-
Interferon-beta therapy reduces CD4+ and CD8+ T-cell reactivity in multiple sclerosis.Immunology. 2007 May;121(1):29-39. doi: 10.1111/j.1365-2567.2006.02518.x. Epub 2006 Dec 18. Immunology. 2007. PMID: 17239199 Free PMC article.
-
Interferon-beta enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis.J Neuroimmunol. 2004 Oct;155(1-2):172-82. doi: 10.1016/j.jneuroim.2004.06.013. J Neuroimmunol. 2004. PMID: 15342209
-
Granulocyte-macrophage colony-stimulating factor as a mediator of autoimmunity in multiple sclerosis.J Neuroimmunol. 2018 Oct 15;323:1-9. doi: 10.1016/j.jneuroim.2018.07.002. Epub 2018 Jul 5. J Neuroimmunol. 2018. PMID: 30196820 Review.
-
The Role of Granulocyte-Macrophage Colony-Stimulating Factor in Murine Models of Multiple Sclerosis.Cells. 2020 Mar 4;9(3):611. doi: 10.3390/cells9030611. Cells. 2020. PMID: 32143326 Free PMC article. Review.
Cited by
-
Correlation between COVID-19 and hepatitis B: A systematic review.World J Gastroenterol. 2022 Dec 14;28(46):6599-6618. doi: 10.3748/wjg.v28.i46.6599. World J Gastroenterol. 2022. PMID: 36569273 Free PMC article.
-
Colony stimulating factors in the nervous system.Semin Immunol. 2021 Apr;54:101511. doi: 10.1016/j.smim.2021.101511. Epub 2021 Nov 4. Semin Immunol. 2021. PMID: 34743926 Free PMC article. Review.
-
Multiple sclerosis.Nat Rev Dis Primers. 2018 Nov 8;4(1):43. doi: 10.1038/s41572-018-0041-4. Nat Rev Dis Primers. 2018. PMID: 30410033 Review.
-
COVID-19 and liver diseases.Egypt Liver J. 2022;12(1):43. doi: 10.1186/s43066-022-00202-2. Epub 2022 Jul 21. Egypt Liver J. 2022. PMID: 35880136 Free PMC article. Review.
-
Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma.Nat Med. 2020 May;26(5):720-731. doi: 10.1038/s41591-020-0827-2. Epub 2020 Apr 27. Nat Med. 2020. PMID: 32341580 Free PMC article.
References
-
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. The New England journal of medicine. 2006;354:942–955. - PubMed
-
- Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. Journal of immunology. 2007;178:39–48. - PubMed
-
- Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nature immunology. 2011;12:560–567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

