Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature
- PMID: 25912949
- PMCID: PMC5079283
- DOI: 10.1016/j.matbio.2015.04.004
Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature
Abstract
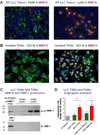
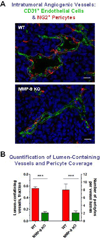
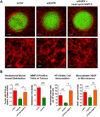
Metastasis is a distinct stage of cancer progression that requires the development of angiogenic blood vessels serving as conduits for tumor cell dissemination. An accumulated body of evidence indicates that metastasis-supporting neovasculature should possess certain structural characteristics allowing for the process of tumor cell intravasation, an active entry of cancer cells into the vessel interior. It appears that the development of tumor vessels with lumens of a distinctive size and support of these vessels by a discontinuous pericyte coverage constitute critical microarchitectural requirements to: (a) provide accessible points for vessel wall penetration by primary tumor cells; (b) provide enough lumen space for a tumor cell or cell aggregate upon intravasation; and (c) allow for sufficient rate of blood flow to carry away intravasated cells from the primary tumor to the next, proximal or distal site. This review will primarily focus on the functional roles of matrix metalloproteinases (MMPs), which catalytically trigger the development of an intravasation-sustaining neovasculature at the early stages of tumor growth and are also required for the maintenance of a metastasis-supporting state of blood vessels at later stages of cancer progression.
Keywords: Epidermal growth factor receptor; Matrix metalloproteinase; Tissue inhibitor of metalloproteinases; Tumor angiogenesis; Tumor cell intravasation and metastasis; Tumor-associated macrophages; Tumor-associated neutrophils; Vascular endothelial growth factor.
Copyright © 2015. Published by Elsevier B.V.
Figures
Similar articles
-
Far Posterior Approach for Rib Fracture Fixation: Surgical Technique and Tips.JBJS Essent Surg Tech. 2024 Dec 6;14(4):e23.00094. doi: 10.2106/JBJS.ST.23.00094. eCollection 2024 Oct-Dec. JBJS Essent Surg Tech. 2024. PMID: 39650795 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
The effectiveness of school-based family asthma educational programs on the quality of life and number of asthma exacerbations of children aged five to 18 years diagnosed with asthma: a systematic review protocol.JBI Database System Rev Implement Rep. 2015 Oct;13(10):69-81. doi: 10.11124/jbisrir-2015-2335. JBI Database System Rev Implement Rep. 2015. PMID: 26571284
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review.
Cited by
-
Neuro-ophthalmic complications of modern anti-cancer drugs.Graefes Arch Clin Exp Ophthalmol. 2024 Jul;262(7):2269-2281. doi: 10.1007/s00417-023-06350-4. Epub 2024 Feb 12. Graefes Arch Clin Exp Ophthalmol. 2024. PMID: 38345654 Free PMC article. Review.
-
Endostar continuous versus intermittent intravenous infusion combined with chemotherapy for advanced NSCLC: a systematic review and meta-analysis including non-randomized studies.BMC Cancer. 2020 Oct 21;20(1):1021. doi: 10.1186/s12885-020-07527-4. BMC Cancer. 2020. PMID: 33087103 Free PMC article.
-
Role of ferroptosis-related molecular patterns in hepatocellular carcinoma microenvironment.Am J Transl Res. 2022 Jan 15;14(1):86-102. eCollection 2022. Am J Transl Res. 2022. PMID: 35173831 Free PMC article.
-
Inhibition of FABP6 Reduces Tumor Cell Invasion and Angiogenesis through the Decrease in MMP-2 and VEGF in Human Glioblastoma Cells.Cells. 2021 Oct 17;10(10):2782. doi: 10.3390/cells10102782. Cells. 2021. PMID: 34685761 Free PMC article.
-
Antiangiogenic, Anti-Inflammatory and Antioxidant Properties of Bidens tripartite Herb, Galium verum Herb and Rumex hydrolapathum Root.Molecules. 2023 Jun 24;28(13):4966. doi: 10.3390/molecules28134966. Molecules. 2023. PMID: 37446627 Free PMC article.
References
-
- Ardi VC, Van den Steen PE, Opdenakker G, Schweighofer B, Deryugina EI, Quigley JP. Neutrophil MMP-9 proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway. J Biol Chem. 2009;284:25854–25866. PCMID2757987. - PMC - PubMed
-
- Bar-Or A, Nuttall RK, Duddy M, Alter A, Kim HJ, Ifergan I, Pennington CJ, Bourgoin P, Edwards DR, Yong VW. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain. 2003;126:2738–2749. - PubMed
-
- Barillari G, Iovane A, Bacigalupo I, Labbaye C, Chiozzini C, Sernicola L, Quaranta MT, Falchi M, Sgadari C, Ensoli B. The HIV protease inhibitor indinavir down-regulates the expression of the pro-angiogenic MT1-MMP by human endothelial cells. Angiogenesis. 2014;17:831–838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01CA55852/CA/NCI NIH HHS/United States
- T32 HL007195/HL/NHLBI NIH HHS/United States
- UL1 RR025774/RR/NCRR NIH HHS/United States
- R01 CA157792/CA/NCI NIH HHS/United States
- R01 CA055852/CA/NCI NIH HHS/United States
- 5T32CA077109/CA/NCI NIH HHS/United States
- UL1 TR000109/TR/NCATS NIH HHS/United States
- R01 CA129484/CA/NCI NIH HHS/United States
- R01CA105412/CA/NCI NIH HHS/United States
- R01CA129484/CA/NCI NIH HHS/United States
- T32 CA077109/CA/NCI NIH HHS/United States
- R01 CA105412/CA/NCI NIH HHS/United States
- UL1 TR000109-05/TR/NCATS NIH HHS/United States
- 5T32HL07195-31/HL/NHLBI NIH HHS/United States
- HL07695/HL/NHLBI NIH HHS/United States
- T32 HL007695/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

