Targeting pancreatitis blocks tumor-initiating stem cells and pancreatic cancer progression
- PMID: 25906749
- PMCID: PMC4558168
- DOI: 10.18632/oncotarget.3499
Targeting pancreatitis blocks tumor-initiating stem cells and pancreatic cancer progression
Abstract
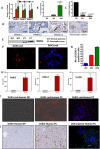
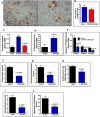
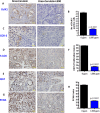
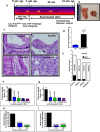
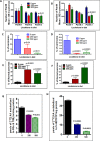
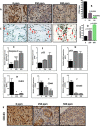
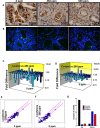
Recent development of genetically engineered mouse models (GEMs) for pancreatic cancer (PC) that recapitulates human disease progression has helped to identify new strategies to delay/inhibit PC development. We first found that expression of the pancreatic tumor-initiating/cancer stem cells (CSC) marker DclK1 occurs in early stage PC and in both early and late pancreatic intraepithelial neoplasia (PanIN) and that it increases as disease progresses in GEM and also in human PC. Genome-wide next generation sequencing of pancreatic ductal adenocarcinoma (PDAC) from GEM mice revealed significantly increased DclK1 along with inflammatory genes. Genetic ablation of cyclo-oxygenase-2 (COX-2) decreased DclK1 in GEM. Induction of inflammation/pancreatitis with cerulein in GEM mice increased DclK1, and the novel dual COX/5-lipoxygenase (5-LOX) inhibitor licofelone reduced it. Dietary licofelone significantly inhibited the incidence of PDAC and carcinoma in situ with significant inhibition of pancreatic CSCs. Licofelone suppressed pancreatic tumor COX-2 and 5-LOX activities and modulated miRNAs characteristic of CSC and inflammation in correlation with PDAC inhibition. These results offer a preclinical proof of concept to target the inflammation initiation to inhibit cancer stem cells early for improving the treatment of pancreatic cancers, with immediate clinical implications for repositioning dual COX/5-LOX inhibitors in human trials for high risk patients.
Keywords: cancer stem cells; dual COX-5-LOX inhibition; inflammation; p48Cre/+-LSL-KrasG12D/+ mice; pancreatic cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Simultaneous targeting of 5-LOX-COX and EGFR blocks progression of pancreatic ductal adenocarcinoma.Oncotarget. 2015 Oct 20;6(32):33290-305. doi: 10.18632/oncotarget.5396. Oncotarget. 2015. PMID: 26429877 Free PMC article.
-
Krüppel-like Factor 5, Increased in Pancreatic Ductal Adenocarcinoma, Promotes Proliferation, Acinar-to-Ductal Metaplasia, Pancreatic Intraepithelial Neoplasia, and Tumor Growth in Mice.Gastroenterology. 2018 Apr;154(5):1494-1508.e13. doi: 10.1053/j.gastro.2017.12.005. Epub 2017 Dec 15. Gastroenterology. 2018. PMID: 29248441 Free PMC article.
-
DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer.Gastroenterology. 2014 Jan;146(1):245-56. doi: 10.1053/j.gastro.2013.09.050. Epub 2013 Oct 2. Gastroenterology. 2014. PMID: 24096005 Free PMC article.
-
Involvement of eicosanoids in the pathogenesis of pancreatic cancer: the roles of cyclooxygenase-2 and 5-lipoxygenase.World J Gastroenterol. 2014 Aug 21;20(31):10729-39. doi: 10.3748/wjg.v20.i31.10729. World J Gastroenterol. 2014. PMID: 25152576 Free PMC article. Review.
-
Protein kinase D1 - A targetable mediator of pancreatic cancer development.Biochim Biophys Acta Mol Cell Res. 2024 Feb;1871(2):119646. doi: 10.1016/j.bbamcr.2023.119646. Epub 2023 Dec 5. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 38061566 Review.
Cited by
-
Modulation of smoke-induced DNA and microRNA alterations in mouse lung by licofelone, a triple COX-1, COX-2 and 5-LOX inhibitor.Carcinogenesis. 2020 Mar 13;41(1):91-99. doi: 10.1093/carcin/bgz158. Carcinogenesis. 2020. PMID: 31562745 Free PMC article.
-
Tepoxalin a dual 5-LOX-COX inhibitor and erlotinib an EGFR inhibitor halts progression of gastric cancer in tumor xenograft mice.Am J Transl Res. 2018 Nov 15;10(11):3847-3856. eCollection 2018. Am J Transl Res. 2018. PMID: 30662635 Free PMC article.
-
Simultaneous targeting of 5-LOX-COX and EGFR blocks progression of pancreatic ductal adenocarcinoma.Oncotarget. 2015 Oct 20;6(32):33290-305. doi: 10.18632/oncotarget.5396. Oncotarget. 2015. PMID: 26429877 Free PMC article.
-
Diabetes Mellitus and Obesity as Risk Factors for Pancreatic Cancer.J Acad Nutr Diet. 2018 Apr;118(4):555-567. doi: 10.1016/j.jand.2017.07.005. Epub 2017 Sep 12. J Acad Nutr Diet. 2018. PMID: 28919082 Free PMC article. Review.
-
Augmented CPT1A Expression Is Associated with Proliferation and Colony Formation during Barrett's Tumorigenesis.Int J Mol Sci. 2022 Oct 4;23(19):11745. doi: 10.3390/ijms231911745. Int J Mol Sci. 2022. PMID: 36233047 Free PMC article.
References
-
- Mazur PK, Siveke JT. Genetically Engineered mouse models of pancreatic cancer: Unravelling tumor biology and progressing translational oncology. Gut. 2012;61:1488–1500. - PubMed
-
- Mohammed A, Janakiram NB, Lightfoot S, Gali H, Vibhudutta A, Rao CV. Early Detection and Prevention of Pancreatic Cancer: Use of Genetically Engineered Mouse Models and advanced Imaging Technologies. Cur Med Chem. 2012;19:3701–3713. - PubMed
-
- Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

