Challenges and advances in the assessment of the disposition of antibody-drug conjugates
- PMID: 25904406
- PMCID: PMC5032988
- DOI: 10.1002/bdd.1957
Challenges and advances in the assessment of the disposition of antibody-drug conjugates
Abstract
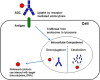
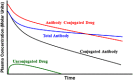
Antibody-drug conjugates (ADCs) are a rapidly growing therapeutic platform for the treatment of cancer. ADCs consist of a cytotoxic small molecule drug linked to an antibody to provide targeted delivery of the cytotoxic agent to the tumor. Understanding the pharmacokinetics (PK) and pharmacodynamics (PD) of ADCs is crucial in their design to optimize dose and regimen, to maximize efficacy and to minimize toxicity in patients. Significant progress has been made in recent years in this area, however, many fundamental questions still remain. This review discusses factors to consider while assessing the disposition of ADCs, and the unique challenges associated with these therapeutics. Current tools that are available and strategies to enable appropriate assessment are also discussed.
Keywords: antibody drug conjugate; biotherapeutics; cancer; pharmacokinetics; preclinical.
© 2015 Genentech Inc. Biopharmaceutics & Drug Disposition Published by John Wiley & Sons Ltd.
Figures
Similar articles
-
Preclinical Pharmacokinetic Considerations for the Development of Antibody Drug Conjugates.Pharm Res. 2015 Nov;32(11):3470-9. doi: 10.1007/s11095-014-1584-z. Epub 2014 Dec 2. Pharm Res. 2015. PMID: 25446773 Free PMC article. Review.
-
Development and Translational Application of an Integrated, Mechanistic Model of Antibody-Drug Conjugate Pharmacokinetics.AAPS J. 2017 Jan;19(1):130-140. doi: 10.1208/s12248-016-9993-z. Epub 2016 Sep 27. AAPS J. 2017. PMID: 27679517
-
Considerations for the nonclinical safety evaluation of antibody drug conjugates for oncology.Regul Toxicol Pharmacol. 2013 Dec;67(3):382-91. doi: 10.1016/j.yrtph.2013.08.017. Epub 2013 Sep 5. Regul Toxicol Pharmacol. 2013. PMID: 24012707
-
Platform model describing pharmacokinetic properties of vc-MMAE antibody-drug conjugates.J Pharmacokinet Pharmacodyn. 2017 Dec;44(6):537-548. doi: 10.1007/s10928-017-9544-y. Epub 2017 Sep 16. J Pharmacokinet Pharmacodyn. 2017. PMID: 28918591
-
Application of Pharmacokinetic-Pharmacodynamic Modeling and Simulation for Antibody-Drug Conjugate Development.Pharm Res. 2015 Nov;32(11):3508-25. doi: 10.1007/s11095-015-1626-1. Epub 2015 Feb 11. Pharm Res. 2015. PMID: 25666843 Review.
Cited by
-
Macrocycle-Antibiotic Hybrids: A Path to Clinical Candidates.Front Chem. 2021 Apr 30;9:659845. doi: 10.3389/fchem.2021.659845. eCollection 2021. Front Chem. 2021. PMID: 33996753 Free PMC article. Review.
-
Bioanalytical Methods and Strategic Perspectives Addressing the Rising Complexity of Novel Bioconjugates and Delivery Routes for Biotherapeutics.BioDrugs. 2022 Mar;36(2):181-196. doi: 10.1007/s40259-022-00518-w. Epub 2022 Apr 1. BioDrugs. 2022. PMID: 35362869 Free PMC article. Review.
-
Prediction of Human Pharmacokinetics of Antibody-Drug Conjugates From Nonclinical Data.Clin Transl Sci. 2019 Sep;12(5):534-544. doi: 10.1111/cts.12649. Epub 2019 Jun 11. Clin Transl Sci. 2019. PMID: 31115997 Free PMC article.
-
Rapid conjugation of antibodies to toxins to select candidates for the development of anticancer Antibody-Drug Conjugates (ADCs).Sci Rep. 2020 Jun 1;10(1):8869. doi: 10.1038/s41598-020-65860-x. Sci Rep. 2020. PMID: 32483228 Free PMC article.
-
A Sequential Population Pharmacokinetic Model of Zilovertamab Vedotin in Patients with Hematologic Malignancies Extrapolated to the Pediatric Population.Clin Pharmacokinet. 2024 Oct;63(10):1489-1499. doi: 10.1007/s40262-024-01429-5. Epub 2024 Oct 22. Clin Pharmacokinet. 2024. PMID: 39438409 Clinical Trial.
References
-
- Sievers EL, Senter PD. Antibody‐drug conjugates in cancer therapy. Annu Rev Med 2013; 64: 15–29. - PubMed
-
- Carter PJ, Senter PD. Antibody‐drug conjugates for cancer therapy. Cancer J 2008; 14(3): 154–169. - PubMed
-
- Mullard A. Maturing antibody‐drug conjugate pipeline hits 30. Nature Rev Drug Discov 2013; 12: 329–332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

