VIH2 Regulates the Synthesis of Inositol Pyrophosphate InsP8 and Jasmonate-Dependent Defenses in Arabidopsis
- PMID: 25901085
- PMCID: PMC4558690
- DOI: 10.1105/tpc.114.135160
VIH2 Regulates the Synthesis of Inositol Pyrophosphate InsP8 and Jasmonate-Dependent Defenses in Arabidopsis
Abstract
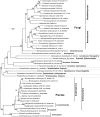
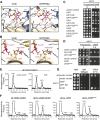
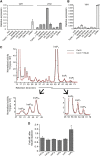
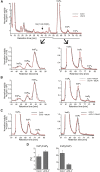
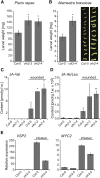
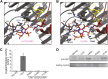
Diphosphorylated inositol polyphosphates, also referred to as inositol pyrophosphates, are important signaling molecules that regulate critical cellular activities in many eukaryotic organisms, such as membrane trafficking, telomere maintenance, ribosome biogenesis, and apoptosis. In mammals and fungi, two distinct classes of inositol phosphate kinases mediate biosynthesis of inositol pyrophosphates: Kcs1/IP6K- and Vip1/PPIP5K-like proteins. Here, we report that PPIP5K homologs are widely distributed in plants and that Arabidopsis thaliana VIH1 and VIH2 are functional PPIP5K enzymes. We show a specific induction of inositol pyrophosphate InsP8 by jasmonate and demonstrate that steady state and jasmonate-induced pools of InsP8 in Arabidopsis seedlings depend on VIH2. We identify a role of VIH2 in regulating jasmonate perception and plant defenses against herbivorous insects and necrotrophic fungi. In silico docking experiments and radioligand binding-based reconstitution assays show high-affinity binding of inositol pyrophosphates to the F-box protein COI1-JAZ jasmonate coreceptor complex and suggest that coincidence detection of jasmonate and InsP8 by COI1-JAZ is a critical component in jasmonate-regulated defenses.
© 2015 American Society of Plant Biologists. All rights reserved.
Figures
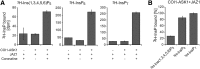

Similar articles
-
Inositol Pyrophosphate InsP8 Acts as an Intracellular Phosphate Signal in Arabidopsis.Mol Plant. 2019 Nov 4;12(11):1463-1473. doi: 10.1016/j.molp.2019.08.002. Epub 2019 Aug 13. Mol Plant. 2019. PMID: 31419530
-
Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor.Nature. 2010 Nov 18;468(7322):400-5. doi: 10.1038/nature09430. Epub 2010 Oct 6. Nature. 2010. PMID: 20927106 Free PMC article.
-
Jasmonic acid perception by COI1 involves inositol polyphosphates in Arabidopsis thaliana.Plant J. 2011 Mar;65(6):949-57. doi: 10.1111/j.1365-313X.2011.04480.x. Epub 2011 Feb 18. Plant J. 2011. PMID: 21205029
-
The JAZ proteins: a crucial interface in the jasmonate signaling cascade.Plant Cell. 2011 Sep;23(9):3089-100. doi: 10.1105/tpc.111.089300. Epub 2011 Sep 30. Plant Cell. 2011. PMID: 21963667 Free PMC article. Review.
-
JAZing up jasmonate signaling.Trends Plant Sci. 2008 Feb;13(2):66-71. doi: 10.1016/j.tplants.2007.11.011. Epub 2008 Feb 7. Trends Plant Sci. 2008. PMID: 18261950 Review.
Cited by
-
How Microbes Twist Jasmonate Signaling around Their Little Fingers.Plants (Basel). 2016 Jan 19;5(1):9. doi: 10.3390/plants5010009. Plants (Basel). 2016. PMID: 27135229 Free PMC article. Review.
-
Analyses of Inositol Phosphates and Phosphoinositides by Strong Anion Exchange (SAX)-HPLC.Methods Mol Biol. 2021;2295:365-378. doi: 10.1007/978-1-0716-1362-7_20. Methods Mol Biol. 2021. PMID: 34047987
-
Quantitative phosphoproteomics reveals molecular pathway network in wheat resistance to stripe rust.Stress Biol. 2024 Jul 1;4(1):32. doi: 10.1007/s44154-024-00170-0. Stress Biol. 2024. PMID: 38945963 Free PMC article.
-
INOSITOL (1,3,4) TRIPHOSPHATE 5/6 KINASE1-dependent inositol polyphosphates regulate auxin responses in Arabidopsis.Plant Physiol. 2022 Nov 28;190(4):2722-2738. doi: 10.1093/plphys/kiac425. Plant Physiol. 2022. PMID: 36124979 Free PMC article.
-
The northern corn leaf blight resistance gene Ht1 encodes an nucleotide-binding, leucine-rich repeat immune receptor.Mol Plant Pathol. 2023 Jul;24(7):758-767. doi: 10.1111/mpp.13267. Epub 2022 Sep 30. Mol Plant Pathol. 2023. PMID: 36180934 Free PMC article.
References
-
- Azevedo C., Saiardi A. (2006). Extraction and analysis of soluble inositol polyphosphates from yeast. Nat. Protoc. 1: 2416–2422. - PubMed
-
- Azevedo C., Burton A., Bennett M., Onnebo S.M., Saiardi A. (2010). Synthesis of InsP7 by the Inositol Hexakisphosphate Kinase 1 (IP6K1). Methods Mol. Biol. 645: 73–85. - PubMed
-
- Blackwell, M., Vilgalys, R., James, T.Y., and Taylor, J.W. (2012). Fungi. Eumycota: mushrooms, sac fungi, yeast, molds, rusts, smuts, etc. In The Tree of Life Web Project, http://tolweb.org/.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

