Astrocyte response to IFN-γ limits IL-6-mediated microglia activation and progressive autoimmune encephalomyelitis
- PMID: 25896970
- PMCID: PMC4410573
- DOI: 10.1186/s12974-015-0293-9
Astrocyte response to IFN-γ limits IL-6-mediated microglia activation and progressive autoimmune encephalomyelitis
Abstract
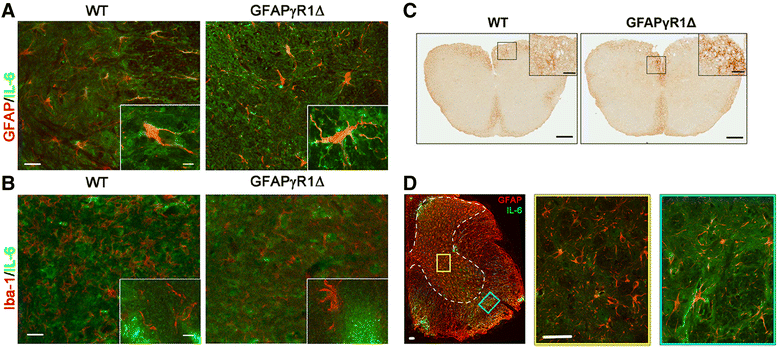
Background: Therapeutic modalities effective in patients with progressive forms of multiple sclerosis (MS) are limited. In a murine model of progressive MS, the sustained disability during the chronic phase of experimental autoimmune encephalomyelitis (EAE) correlated with elevated expression of interleukin (IL)-6, a cytokine with pleiotropic functions and therapeutic target for non-central nervous system (CNS) autoimmune disease. Sustained IL-6 expression in astrocytes restricted to areas of demyelination suggested that IL-6 plays a major role in disease progression during chronic EAE.
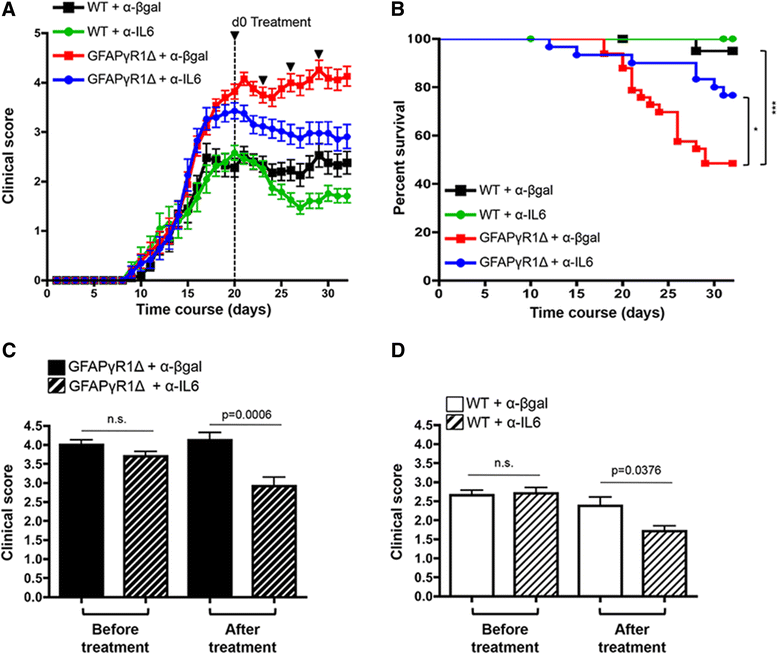
Methods: A progressive form of EAE was induced using transgenic mice expressing a dominant negative interferon-γ (IFN-γ) receptor alpha chain under control of human glial fibrillary acidic protein (GFAP) promoter (GFAPγR1Δ mice). The role of IL-6 in regulating progressive CNS autoimmunity was assessed by treating GFAPγR1Δ mice with anti-IL-6 neutralizing antibody during chronic EAE.
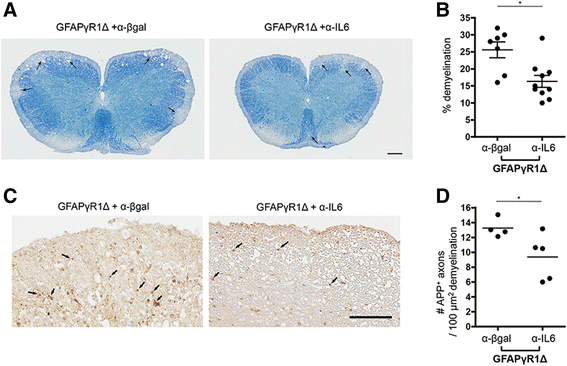
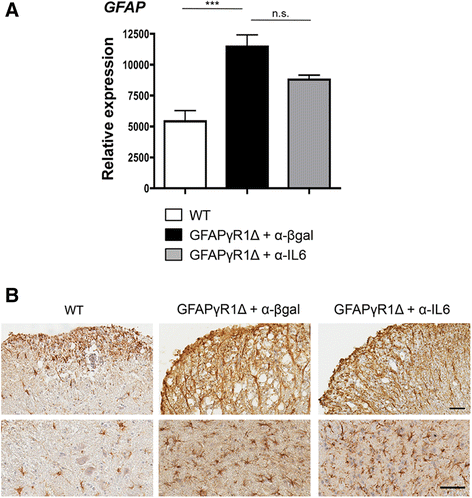
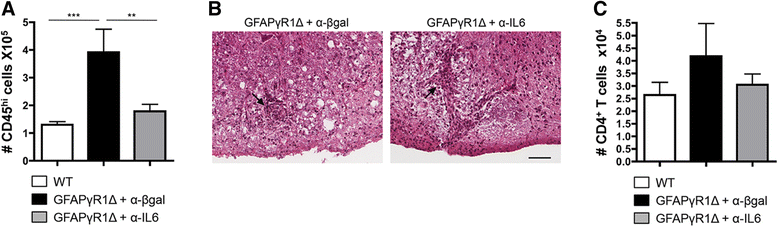
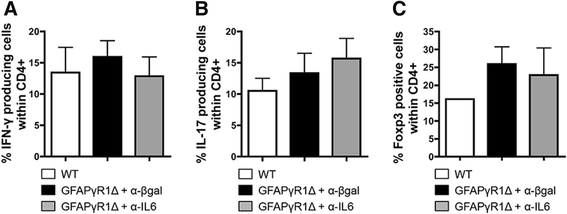
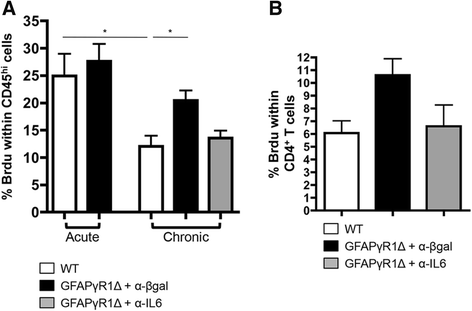
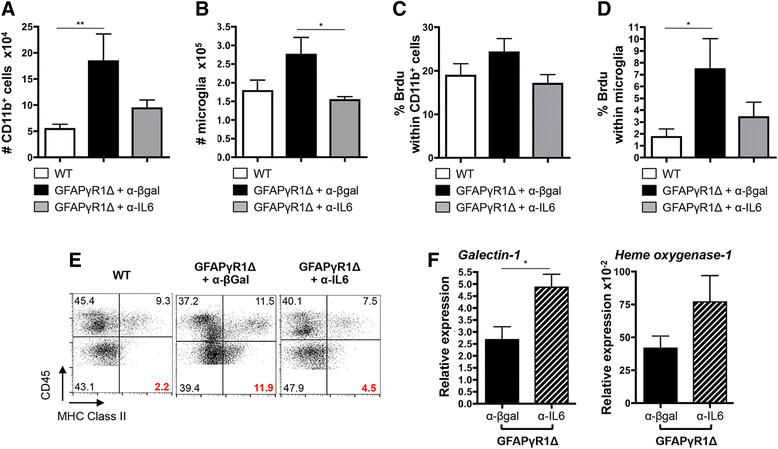
Results: IL-6 neutralization restricted disease progression and decreased disability, myelin loss, and axonal damage without affecting astrogliosis. IL-6 blockade reduced CNS inflammation by limiting inflammatory cell proliferation; however, the relative frequencies of CNS leukocyte infiltrates, including the Th1, Th17, and Treg CD4 T cell subsets, were not altered. IL-6 blockade rather limited the activation and proliferation of microglia, which correlated with higher expression of Galectin-1, a regulator of microglia activation expressed by astrocytes.
Conclusions: These data demonstrate that astrocyte-derived IL-6 is a key mediator of progressive disease and support IL-6 blockade as a viable intervention strategy to combat progressive MS.
Figures
Similar articles
-
Sustained TNF production by central nervous system infiltrating macrophages promotes progressive autoimmune encephalomyelitis.J Neuroinflammation. 2016 Feb 22;13:46. doi: 10.1186/s12974-016-0513-y. J Neuroinflammation. 2016. PMID: 26906225 Free PMC article.
-
Gp130-dependent astrocytic survival is critical for the control of autoimmune central nervous system inflammation.J Immunol. 2011 Jun 1;186(11):6521-31. doi: 10.4049/jimmunol.1001135. Epub 2011 Apr 22. J Immunol. 2011. PMID: 21515788
-
Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis.Brain Behav Immun. 2010 May;24(4):641-51. doi: 10.1016/j.bbi.2010.01.014. Epub 2010 Feb 6. Brain Behav Immun. 2010. PMID: 20138983
-
Identification of new therapeutic targets for prevention of CNS inflammation.Expert Opin Ther Targets. 2002 Apr;6(2):203-15. doi: 10.1517/14728222.6.2.203. Expert Opin Ther Targets. 2002. PMID: 12223081 Review.
-
Anti-inflammatory immunotherapy for multiple sclerosis/experimental autoimmune encephalomyelitis (EAE) disease.Curr Med Chem. 2005;12(25):2947-62. doi: 10.2174/092986705774462833. Curr Med Chem. 2005. PMID: 16378498 Review.
Cited by
-
Drug repurposing based on the similarity gene expression signatures to explore for potential indications of quercetin: a case study of multiple sclerosis.Front Chem. 2023 Sep 8;11:1250043. doi: 10.3389/fchem.2023.1250043. eCollection 2023. Front Chem. 2023. PMID: 37744058 Free PMC article.
-
Overview of diet and autoimmune demyelinating optic neuritis: a narrative review.Immunometabolism (Cobham). 2023 Apr 27;5(2):e00022. doi: 10.1097/IN9.0000000000000022. eCollection 2023 Apr. Immunometabolism (Cobham). 2023. PMID: 37128292 Free PMC article. Review.
-
Microglia and Brain Disorders: The Role of Vitamin D and Its Receptor.Int J Mol Sci. 2023 Jul 25;24(15):11892. doi: 10.3390/ijms241511892. Int J Mol Sci. 2023. PMID: 37569267 Free PMC article. Review.
-
Extracellular vesicles (exosomes and ectosomes) play key roles in the pathology of brain diseases.Mol Biomed. 2021 Jun 20;2(1):18. doi: 10.1186/s43556-021-00040-5. Mol Biomed. 2021. PMID: 35006460 Free PMC article. Review.
-
Interleukin-6 Derived from the Central Nervous System May Influence the Pathogenesis of Experimental Autoimmune Encephalomyelitis in a Cell-Dependent Manner.Cells. 2020 Jan 31;9(2):330. doi: 10.3390/cells9020330. Cells. 2020. PMID: 32023844 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

